
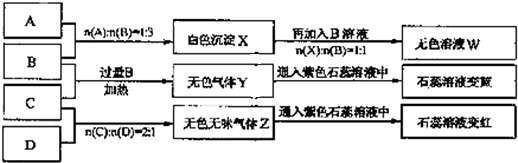
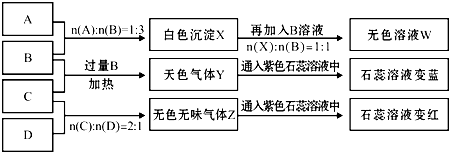
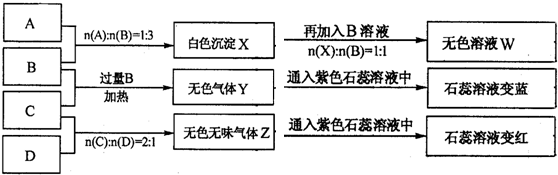
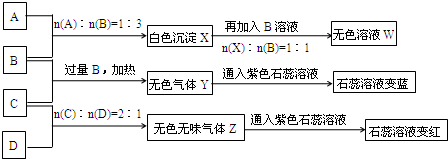
��10�֣�������ƿ��ʧ��ǩ��NaOH��Na2CO3��AlCl3��NH4HSO4��Һ��Ϊ������ƿ��Һ������ƿ��Һ���ΪA��B��C��D����ʵ�顣ʵ����̺ͼ�¼����ͼ��ʾ���������Ѿ���ȥ����
��ش�
��1��A��W����Һ�ֱ�������ɲ�����������ù���Ϊ �� ���ѧʽ����
��2��D��ҺpH ���������������������7��ԭ���ǣ������ӷ���ʽ��ʾ�� ��
��3�������ʵ���Ũ�ȵ�A��B��C��D��ҺpH�ɴ�С��˳����NaOH> .> >
NH4HSO4�����û�ѧʽ��ʾ��
��4�������ʵ�Ũ�ȵ�C��Һ��NH4Cl��Һ��Ƚϣ�c��NH4����ǰ�� ���ߣ����������������������
��5������ϡ��Һ��B��C�����ʵ���֮��2��1��Ӧ�������ӷ���ʽΪ
(1)Al2O3 �� NaAlO2 (2)���ڣ� CO32-+H2O HCO3-+OH-
HCO3-+OH-
(3)NaOH>Na2CO3>AlCl3>NH4HSO4
(4) �� (5) NH4++H++2OH-=NH3��H2O+H2O
�������������A��B�����ʵ���1��3�������ɰ�ɫ�������ټ���B�����ܽ⣬��֪B��NaOH��Һ��A��AlCl3��Һ��B��C�����ܲ�����ɫ���壬���������ʹʯ����Һ������˵��C��NH4HSO4��Һ����D��Na2CO3��Һ�����X��Al(OH)3��Y��NH3��Z��CO2��W��NaAlO2��Һ����1��A�ǻӷ�����������Σ������������պ�õ�������Al2O3��W���ѻӷ�����������Σ������������պ�õ�ԭ����NaAlO2 ����2��Na2CO3ǿ�������Σ���Һ�Լ��ԣ�ԭ����CO32-+H2O HCO3-+OH-����3��AlCl3ǿ�������Σ���Һ�����ԣ�NaOHǿ���Һ�Լ��ԣ�NH4HSO4ǿ�����ʽ�Σ��൱��һԪǿ�ᣩ����Һ�����ԣ�Na2CO3ǿ�������Σ���Һ�Լ��ԣ����Ե�Ũ�ȵ�AlCl3��Һ��NaOH��Һ��NH4HSO4��Һ��Na2CO3��ҺpH�ɴ�С��˳����NaOH>Na2CO3>AlCl3>NH4HSO4����4��NH4HSO4��Һ���������H+����NH4��ˮ�⣬����c(NH4)��5��NaOH��NH4HSO4��2��1��Ӧ�����ӷ���ʽΪ��NH4++H++2OH-=NH3��H2O+H2O��
HCO3-+OH-����3��AlCl3ǿ�������Σ���Һ�����ԣ�NaOHǿ���Һ�Լ��ԣ�NH4HSO4ǿ�����ʽ�Σ��൱��һԪǿ�ᣩ����Һ�����ԣ�Na2CO3ǿ�������Σ���Һ�Լ��ԣ����Ե�Ũ�ȵ�AlCl3��Һ��NaOH��Һ��NH4HSO4��Һ��Na2CO3��ҺpH�ɴ�С��˳����NaOH>Na2CO3>AlCl3>NH4HSO4����4��NH4HSO4��Һ���������H+����NH4��ˮ�⣬����c(NH4)��5��NaOH��NH4HSO4��2��1��Ӧ�����ӷ���ʽΪ��NH4++H++2OH-=NH3��H2O+H2O��
���㣺���ƶ���
���������������ƶ������ӷ�Ӧ������ˮ����ۺ����ã��ۺ��Խ�ǿ���Ѷ����У����Զ���ѧ������˼ά������


 ���Ͱ�ͨ�������Сѧ��ʱͬ�����ϵ�д�
���Ͱ�ͨ�������Сѧ��ʱͬ�����ϵ�д� ���Ͱ�ͨ������ϵ�д�
���Ͱ�ͨ������ϵ�д� �ٷ�ѧ����ҵ��������ϵ�д�
�ٷ�ѧ����ҵ��������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| ||
| ||
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

��������и��⣺
(1)Y��Z�Ļ�ѧʽ�ֱ�ΪY___________��Z___________��X��B��Ӧ�����ӷ���ʽΪ____________________��
(2)D��ҺpH______________7(����ڡ���С�ڡ����ڡ�)��ԭ����(�����ӷ���ʽ��ʾ)____________________________________________________________________��
(3)�����ʵ���Ũ�ȵ�A��B��C��D��ҺpH�ɴ�С��˳����____________________��(�û�ѧʽ��ʾ)
(4)��д��C�����B��Ӧ(����)�����ӷ���ʽ____________________��
(5)��B��C��ϡ��Һ��Ϻ�(������)��Һ�����ԣ������Һ������Ũ�ȴӴ�С��˳����____________________��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com