
������ˮ�п��ܴ��ڵ���ƽ�⡢�ε�ˮ��ƽ��ͳ������ܽ�ƽ�⣬���Ƕ��ɿ�����ѧƽ�⡣�������ѧ֪ʶ�ش�
��1����0.1 mol��L��1��(NH4)2SO4��Һ�У��������ӵ�Ũ���ɴ�С˳��Ϊ________��
��2��ʵ����0.1 mol��L��1NaHCO3��Һ��pH��7����ӵ����ˮ���������������NaHCO3��Һ�Լ��Ե�ԭ��_____________________________________�����û�ѧ���P������ֻش�
��3������ͨ����ĭ������ڵ�����Ͳ��ʢ��Al2(SO4)3��Һ����Ͳ��ʢ��NaHCO3��Һ��������ԭ����__________________�����û�ѧ���P������ֻش�
�ڲ��ܰ�Al2(SO4)3��Һʢ������Ͳ�е�ԭ����___________�����û�ѧ���P������ֻش𣩢۲����ܽ�Ƚϴ��Na2CO3����NaHCO3��Һ��ԭ����___________________________��

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ��2017�����ʡ�����и����ڶ����ʼ컯ѧ�Ծ��������棩 ���ͣ�ѡ����
���й����л��������˵����ȷ����
A. 2-������Ҳ���춡��
B. ��Ԫ��ϩ( )�뱽��Ϊͬ���칹��
)�뱽��Ϊͬ���칹��
C. C4H9Cl��3��ͬ���칹��
D. ����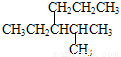 ����ȷ������2-��-3-��������
����ȷ������2-��-3-��������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2017�����ʡ���У��ݣ�������һ�����ϵ�����3�������������ۺϻ�ѧ�Ծ��������棩 ���ͣ�ѡ����
25��ʱ����10 mL 0.1 mol��L��1 H2A��Һ�еμӵ�Ũ�ȵ�NaOH��Һ����Һ��pH��NaOH��Һ�������ϵ��ͼ��ʾ�����������������
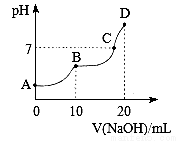
A. C����Һ�к���NaHA��Na2A
B. NaHA��Һ��ˮ�ĵ���̶ȱ�Na2A��Һ��С
C. B�㣬c (Na��)��2[c (H2A)��c (HA)��c (A2��)]
D. D�㣬c (Na��)��c (A2��)��c (OH��)��c (HA��)��c (H��)
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2016-2017ѧ��㶫ʡտ���и߶���ѧ����ĩ���п��Ի�ѧ�Ծ��������棩 ���ͣ�ѡ����
�ö��Ե缫ʵ�ֵ�⣬����˵����ȷ����
A. ���ϡ���ᣬʵ���ǵ��ˮ����ҺpH����
B. �����������ϡ��Һ����ҺŨ������pH��С
C. ����Ȼ�����Һ����ҺŨ�ȼ�С��pH����
D. �������ͭ��Һ��Ҫ����OH������ҺpH��С
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2016-2017ѧ��㶫ʡտ���и߶���ѧ����ĩ���п��Ի�ѧ�Ծ��������棩 ���ͣ�ѡ����
�����й�ʵ�������˵����ȷ����
A. ��10mL��Ͳ��ȡ8.10mLϡ����
B. ����25mL��ʽ�ζ�����ȡ20.00mL KMnO4��Һ
C. ��pH��ֽ�ⶨ��Һ��pHʱ������������ˮ��ʪ��ֽ
D. ��pH�Ʋ��ij��Һ��pHΪ2
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2016-2017ѧ�긣��ʡ�����и߶���ѧ����ĩ���Ի�ѧ�Ծ��������棩 ���ͣ�ѡ����
�����£���0.1000 mol��L��1 HCl��Һ�ζ�20.00 mL 0.100 0 mol��L��1 NH3��H2O��Һ���ζ���������ͼ������˵������ȷ����

A. ����Һ��c(NH3��H2O)+ c(NH )��2c(Cl��)
)��2c(Cl��)
B. ����Һ��c(NH )��2c(H��)��2c(OH��)��c(NH3��H2O)
)��2c(H��)��2c(OH��)��c(NH3��H2O)
C. ����Һ��c(NH )��c(Cl��)>c(OH��)��c(H��)
)��c(Cl��)>c(OH��)��c(H��)
D. ����Һ��c(NH ) > c(Cl��) > c(H��) > c(OH��)
) > c(Cl��) > c(H��) > c(OH��)
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2016-2017ѧ�긣��ʡ�����и߶���ѧ����ĩ���Ի�ѧ�Ծ��������棩 ���ͣ�ѡ����
���ڿ��淴Ӧ��2AB3(g) A2(g)��3B2(g)����H>0������ͼ������ȷ����
A2(g)��3B2(g)����H>0������ͼ������ȷ����
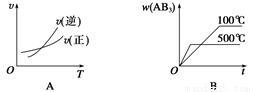

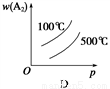
A. A B. B C. C D. D
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2016-2017ѧ��ӱ�ʡ�żҿ��и߶���ѧ����ĩ���Ի�ѧ�Ծ��������棩 ���ͣ�ѡ����
�����£������ʵ���Ũ����ȵ��������ʵ���Һ����NH4NO3 ��CH3COONH4 ��NH4HSO4 �ܣ�NH4��2SO4 �ݣ�NH4��2CO3������������c��NH4�����ɴ�С��˳����
A. �ڢ٢ۢݢ� B. �٢ڢۢܢ� C. �ܢݢۢ٢� D. �ݢܢۢڢ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2016-2017ѧ��ӱ�ʡ�żҿ��и�һ��ѧ����ĩ���Ի�ѧ�Ծ��������棩 ���ͣ�ѡ����
���й��ڽ����˵����ȷ����
A. ֱ������1��100����֮�������Ϊ����
B. ������ͨ������ʱ�������ġ������������ڹⱻ��������ɢ����γɵ�
C. ����ķ�ɢ�ʲ�������ֽ�Ŀ�϶
D. ��Һ�ǵ����Եģ������Ǵ����
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com