
����Ŀ����ѧ��������¹�ֽⷨ�Ʊ�������
��2Ce4��(aq)��H2O(l)===2Ce3��(aq)��![]() O2(g)��2H��(aq)����H1
O2(g)��2H��(aq)����H1
��Ce3��(aq)��H2O(l)===Ce4��(aq)��![]() H2(g)��OH��(aq)����H2
H2(g)��OH��(aq)����H2
��H2O(l)===H��(aq)��OH��(aq)����H3
��2H2O(l)===2H2(g)��O2(g)����H4
����˵����ȷ����(����)
A. Ce4���ܹ�����ˮ�ֽⷴӦ�Ļ�ܣ���߷�Ӧ����
B. Ce3���Ƿ�Ӧ�ںͷ�Ӧ�۵Ĵ���
C. ������Ӧ�У���H4��2��H1��4��H2��4��H3
D. ͨ�������£���Ӧ��������H2��O2������֮��Ϊ1��2
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����A��B��C��D��E���ֶ�����Ԫ�أ���֪���ڵ�A��B��C��D����Ԫ��ԭ�Ӻ�������㹲��24�����ӣ������ڱ��е�λ����ͼ��ʾ��E�ĵ��ʿ����ᷴӦ��1molE���������������ã��ڱ�״�����ܲ���33.6LH2��E����������A�������Ӻ�����Ӳ�ṹ��ȫ��ͬ
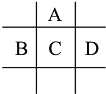
�ش��������⣺
(1) A��E�γɵĻ�����Ļ�ѧʽ��__________��B����������ﻯѧʽΪ_____________��
(2)D�ĵ�����ˮ��Ӧ�ķ���ʽΪ__________________________________________________��
(3) ��D��E�γɵĻ������ˮ��Һ�е����ռ���Һֱ���������۲쵽��������___________________���йط�Ӧ�����ӷ���ʽΪ��_____________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�������������ʣ�
��ϡ���� ��С�մ� �۰�ˮ �ܶ�����̼ ��FeCl3���� ��ϡNaOH��Һ ������������Һ
��1���������������ڵ���ʵ��������Ϊ______ ��
��2���������ᡢNaOH��Һ���ܷ�����ѧ��Ӧ���������Ϊ______��
��3��д��С�մ���θ�ᣨ��Ҫ�ɷ�Ϊϡ���ᣩ���õ����ӷ���ʽ��___________
��4��ʵ�����â��Ʊ�����Ļ�ѧ����ʽΪ_____________________________�������1molFeCl3ȫ���Ƴɽ��壬�ƵõĽ���________(ѡ������硱�������ԡ��������硱)��������Ŀ________NA(ѡ����ڡ������ڡ���С�ڡ�)
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���������ʼ��ת��������ͨ��һ����Ӧʵ�ֵ���( )
A.Si��K2SiO3B.Na��Na2O2C.Fe��FeC12D.CuO��Cu(OH)2
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����֪H2O2�ڴ��������·ֽ����ʼӿ죬�������淴Ӧ���̵ı仯����ͼ��ʾ������˵����ȷ����(����)
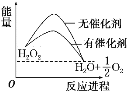
A. �����������С�˷�Ӧ����ЧӦ
B. ��������������H2O2��ƽ��ת����
C. H2O2�ֽ���Ȼ�ѧ����ʽ��H2O2===H2O��1/2 O2����H>0
D. ��Ӧ��������������������������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���ʻ���COS����ȫ����ѭ������Ҫ�м��壬Ҳ���л��ϳ��е���Ҫԭ�ϣ��ǻ�ѧ��������Ҫ���о�������֪��
����COS��g����H2��g��![]() H2S��g����CO��g�� ��Hl����17kJ��mol��
H2S��g����CO��g�� ��Hl����17kJ��mol��
����COS��g����H2O��g��![]() H2S��g����CO2��g�� ��H2����35kJ��mol��
H2S��g����CO2��g�� ��H2����35kJ��mol��
�ش��������⣺
(1)��ӦCO��g����H2O��g��![]() H2��g����CO2��g���ġ�H��________��
H2��g����CO2��g���ġ�H��________��
(2)�ڳ��д����ĺ�ѹ�ܱ������н��з�ӦI������ʼ�����n��H2����n��COS����m����ͬʱ���ڲ��COSת������m���¶ȣ�T���Ĺ�ϵ��ͼ��ʾ��
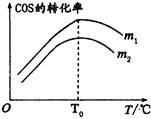 ��m1________m2�����������
��m1________m2�����������
���¶ȸ���T0ʱ��COSת���ʼ�С�Ŀ���ԭ��Ϊ_________��
A���и���Ӧ������
B����Ӧ�ġ�H����
C���������Խ��͡�
D���淴Ӧ��������ı���С������Ӧ��������ı���
(3)�ں��¡������ܱ������У����з�ӦI������˵������˵����ӦI�Ѵﵽƽ��״̬����___________��
A��c��H2S����c��CO�� B��v����H2����v����H2S��
C�������������ܶȱ��ֲ��� D�������л������ƽ��Ħ���������ֲ���
E��c��COS�����ֲ���
(4)ij�¶��£������Ϊ2 L�ĺ����ܱ�������ͨ��5 mol COS��g����5 molH2O��g����������Ӧ����5 min��Ӧ�ﵽƽ�⣬���COS��g����ת����Ϊ80����
�ٷ�Ӧ����ʼ��5 min�ڣ���H2SŨ�ȱ仯��ʾ��ƽ����Ӧ�ٶ�v��H2S����________��
�ڸ��¶��£�������Ӧ��ƽ�ⳣ��K��________��
������������ͬʱ������ʹ������Ӧ��COS��ƽ��ת���ʽ��ͣ�����ʹ��Ӧ���ʼӿ��������________��
A����С�����ݻ� B�������¶�
C����������� D������һ����H2O��g��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������ʱ����һ��ƺʹ���ʹ��ζ��ɿڣ�ԭ���ǣ� ��
A. �������������� B. ��������������
C. ����������� D. ��������������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����Ҫ��ش��������⣺
��1��д��������̼�ĵ���ʽ________________��
��2������CH3CH2CH2CH(CH3)2������__________________��
��3����Ƭ��ͭƬ���Ӻ����ϡ�����γ�ԭ��أ�������Ӧʽ��_________________��
��4���Ҵ�����ֵΪQ kJ��g-1��д���Ҵ���ȫȼ������CO2�����Һ̬ˮ���Ȼ�ѧ����ʽ______________��
��5��д������Ũ���ᡢŨ������55���·�Ӧ�Ļ�ѧ����ʽ____________________��
��6����X������̼�����������Ϊ36��7����ѧ������������ƣ�����X��ͬ���칹�干��________�֡�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������̪���ҹ������з���һ���������Ƽ���ȱѪ���������ҩ���ϳɶ���̪��J����һ��·����ͼ��ʾ��


��1��A��������_______��E��F�ķ�Ӧ������___________��
��2���Լ�a��________��F�й�����������_______��
��3��M��ɱ�F��1��CH2��M�ķ���ʽΪC8H7BrO��M��ͬ���칹���У����ܷ���������Ӧ���ں��б������۲���������������������M��ͬ���칹�干��______�֡�
��4��J��һ�����������г����������һ����Ԫ����J�Ľṹ��ʽΪ__________��H��һ�������»������ɸ߷��ӻ�����K��H����K�Ļ�ѧ����ʽΪ________��
��5������������Ϣд������ȩ�ͱ�Ϊԭ�ϣ��ϳ�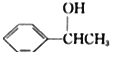 ��·������ͼ�������Լ���ѡ����________��
��·������ͼ�������Լ���ѡ����________��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com