��2009?������ij�о���С�����A-D������װ������й�ʵ��
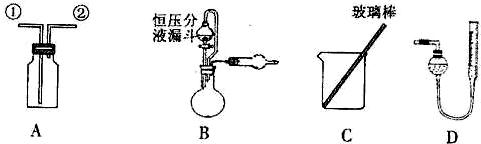
��ʵ��һ���ռ�NO���壮
��1����װ��A�ռ�NO���壬��ȷ�IJ�����
C
C
������ţ���
a���Ӣٿڽ���������ˮ������ b���Ӣٿڽ�����������������
c���Ӣڿڽ���������ˮ������ d���Ӣڿڽ�����������������
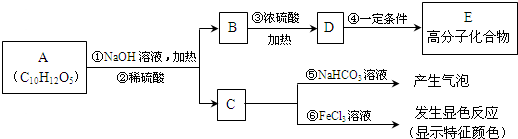
��ʵ�����Ϊ��̽����п�������ϵ�п������������Zn�ͶƲ��ȣ���ѯ��֪п�����ڼZn+2NaOH=Na
2ZnO
3+H
2���ݴˣ���ȡ���ΪS��˫���п���������������顢�Ƶ�����Ϊm
1g���ù����ռ��ˮ���Լ����������ʵ�鷽�����������ʵ�飮
�����ף�ͨ������������Ӧ���ɵ����������ʵ��̽��ľĿ�꣮
��2��ѡ��B��
D
D
����������ţ�����װ�ý���ʵ�飮
��3����ó�ַ�Ӧ���������������ΪVL����״�������أ�Zn���T
��
��4������Ʋ��ȣ�����Ҫ������һ����������
����п���ܶ�
����п���ܶ�
��
��5����װ��B�еĺ�ѹ��Һ©����Ϊ��ͨ��Һ©�������������
ƫ��
ƫ��
���ƫ����ƫС������Ӱ�족����
�����ң�ͨ������������Ӧǰ�������ʵ��̽��Ŀ�꣮ѡ������C��ʵ�飬��������ַ�Ӧ���˳������ϴ�ӡ���ɣ��Ƶ�������Ϊm
2g��
��6���أ�Zn��=
��
��������ͨ������������Ӧǰ���������������Լ��������������ֵ��ΪH
2��������ʵ��̽��Ŀ�꣮ʵ��ͬ��ʹ������C��
��7����ʵ�����Ƕȷ�����������
����
����
�����ң�����ڡ��������ڡ���ͬ�ڡ�����

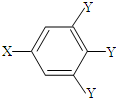 �����У�-X��-Y��Ϊ�����ţ���
�����У�-X��-Y��Ϊ�����ţ���



 +NaHCO3��
+NaHCO3��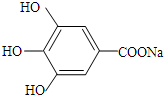 +H2O+CO2��
+H2O+CO2�� +NaHCO3��
+NaHCO3�� +H2O+CO2��
+H2O+CO2��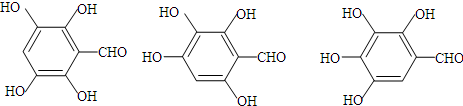 ��д������3���ṹ��ʽ�е�����2�����ɣ�
��д������3���ṹ��ʽ�е�����2�����ɣ� ��д������3���ṹ��ʽ�е�����2�����ɣ�
��д������3���ṹ��ʽ�е�����2�����ɣ� ��B��C��A��������ˮ�ⷴӦ�õ��IJ������A�Ľṹ��ʽΪ��
��B��C��A��������ˮ�ⷴӦ�õ��IJ������A�Ľṹ��ʽΪ�� ��
�� ���ʴ�Ϊ���Ȼ���
���ʴ�Ϊ���Ȼ��� ��
�� ��B��C��A��������ˮ�ⷴӦ�õ��IJ������A�Ľṹ��ʽΪ��
��B��C��A��������ˮ�ⷴӦ�õ��IJ������A�Ľṹ��ʽΪ�� ���ʴ�Ϊ��
���ʴ�Ϊ�� ��
�� ��̼�����Ʒ�����Ӧ��ԭ������ʽΪ��
��̼�����Ʒ�����Ӧ��ԭ������ʽΪ�� +NaHCO3��
+NaHCO3�� +H2O+CO2�����ʴ�Ϊ��
+H2O+CO2�����ʴ�Ϊ�� +NaHCO3��
+NaHCO3�� +H2O+CO2����
+H2O+CO2���� ���ʴ�Ϊ��
���ʴ�Ϊ�� ��д������3���ṹ��ʽ�е�����2�����ɣ���
��д������3���ṹ��ʽ�е�����2�����ɣ���

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
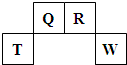 ��2009?������������Ԫ��Q��R��T��W��Ԫ�����ڱ��е�λ����ͼ��ʾ������T��������������������������ȣ�
��2009?������������Ԫ��Q��R��T��W��Ԫ�����ڱ��е�λ����ͼ��ʾ������T��������������������������ȣ�


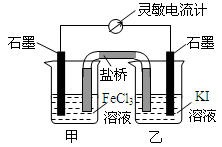 ��2009?�����������ʺϵ�����������Ӧ2Fe3++2I-?2Fe2++I2��Ƴ�����ͼ��ʾ��ԭ��أ������жϲ���ȷ���ǣ�������
��2009?�����������ʺϵ�����������Ӧ2Fe3++2I-?2Fe2++I2��Ƴ�����ͼ��ʾ��ԭ��أ������жϲ���ȷ���ǣ�������