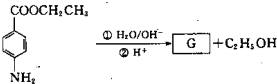
[��ѧ--�л���ѧ����]
���A��C
6H
8O
4��ΪʳƷ��װֽ�ij��÷�������A����ʹ��ˮ��ɫ��A������ˮ���������������¿ɷ���ˮ�ⷴӦ���õ�B��C
4H
4O
4���ͼ״���ͨ��״����BΪ��ɫ���壬��������������Һ������Ӧ��
��1��A���Է����ķ�Ӧ��
�٢ۢ�
�٢ۢ�
��ѡ����ţ���
�ټӳɷ�Ӧ ��������Ӧ �ۼӾ۷�Ӧ ��������Ӧ
��2��B�������������ŵ�������
̼̼˫��
̼̼˫��
��
�Ȼ�
�Ȼ�
��
��3��B������û��֧������ṹ��ʽ��
HOOCCH=CHCOOH
HOOCCH=CHCOOH
��B�ľ�����ͬ�����ŵ�ͬ���칹��Ľṹ��ʽ��
CH2=C��COOH��2
CH2=C��COOH��2
��
��4����B��ȡA�Ļ�ѧ����ʽ��
HOOCCH=CHCOOH+2CH
3OH
CH
3OOCCH=CHCOOCH
3+2H
2O
HOOCCH=CHCOOH+2CH
3OH
CH
3OOCCH=CHCOOCH
3+2H
2O
��
��5�����Ŷ����ᣨC
4H
7NO
4����������嵰���ʵİ�����֮һ������Bͨ�����·�Ӧ��ȡ��
���Ŷ�����Ľṹ��ʽ��
HOOCCH2CH��NH2��COOH
HOOCCH2CH��NH2��COOH
��




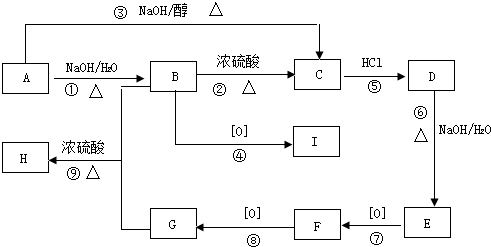
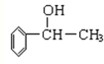 [��ѧ--�л���ѧ����]
[��ѧ--�л���ѧ����]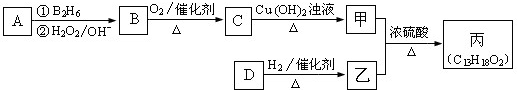
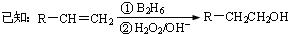
 -CH=CHCHO
-CH=CHCHO -CH=CHCHO
-CH=CHCHO -CH2CH2CH2OH
-CH2CH2CH2OH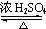 ��CH3��2CHCOOCH2CH2CH2-
��CH3��2CHCOOCH2CH2CH2- +H2O
+H2O -CH2CH2CH2OH
-CH2CH2CH2OH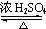 ��CH3��2CHCOOCH2CH2CH2-
��CH3��2CHCOOCH2CH2CH2- +H2O
+H2O

 �������ڵ��ƾ��в�ݮ�����ѡ�ӣ�ҡ�����������ζ��ʳ���㾫��
�������ڵ��ƾ��в�ݮ�����ѡ�ӣ�ҡ�����������ζ��ʳ���㾫��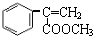
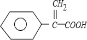 +CH3OH
+CH3OH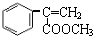 +H2O
+H2O +CH3OH
+CH3OH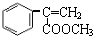 +H2O
+H2O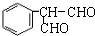
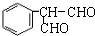
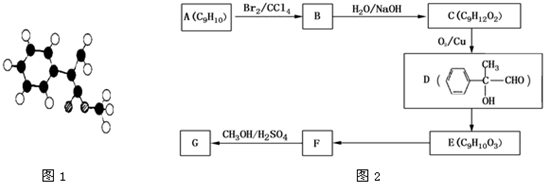
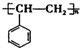
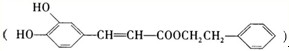 ��һ����Ȼ����ҩ���һ���������ܷ�������ת����
��һ����Ȼ����ҩ���һ���������ܷ�������ת����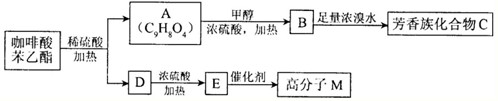



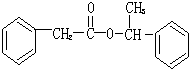
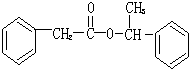
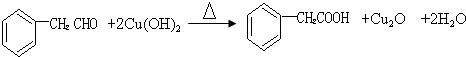

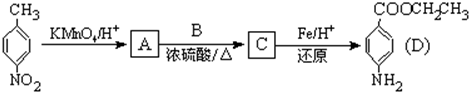
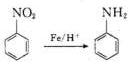
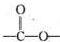 �ṹ�Ļ�����������
�ṹ�Ļ�����������