
��֪AΪ��ʽ�Σ�BΪij���������������ԭ������������20����һ����������³�ѹ��C��D��F��G��I������̬�������ʵ���A��B��ֻ�Ϻ������ǡ����ȫ��Ӧ��ͼ�з�Ӧ�������������⣩������ȥ��

��1��д��A�Ļ�ѧʽ ��B�ĵ���ʽ ��I�Ľṹʽ ��
��2��д�����з�Ӧ�����ӷ���ʽ��
����A��Һ�м���M ��
����A��Һ�м������NaOH��Һ�������� ��
��3���������G���м�ѹ������������������ ��
������Ӧ�Ļ�ѧ����ʽ�� ��

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

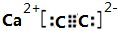
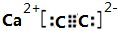
| ||
| ||
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
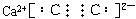
(1)д��B�ĵ���ʽ___________��D�Ľṹ��ʽ___________��
(2)д��A��B��Ӧ�Ļ�ѧ����ʽ____________________________________��
(3)��I����ͨ�����б���C�����NaCl��Һ���Ǻ����Ƽ��һ���ؼ���Ӧ���÷�Ӧ�Ļ�ѧ����ʽΪ___________________________________��
(4)д�����з�Ӧ�����ӷ���ʽ��
����A��Һ�м���M_________________________________��
����A��Һ�м������NaOH��Һ��������________________________��
����֪Fe3O4������M��Һ������F���壬�����ӷ���ʽΪ_______________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��֪AΪ��ʽ�Σ�BΪij�����������Ԫ�صĻ�������³�ѹ��C��D��F��G��I������̬�������ʵ���A��B��������ˮ��ֻ����ǡ����ȫ��Ӧ��ͼ�з�Ӧ�������������⣩������ȥ��

��1��д��B�ĵ���ʽ ��D�Ľṹ��ʽ ________��
��2��д��A��B��Ӧ�Ļ�ѧ����ʽ ��
��3���������G���м�ѹ������������������ ��
��4��д�����з�Ӧ�����ӷ���ʽ��
�� ��A��Һ�м���M ��
�� ��A��Һ�м������NaOH��Һ�������� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��12�֡���֪AΪ��ʽ�Σ�BΪij�����������Ԫ�صĻ����C��D��F��G��I�ڳ��³�ѹ�¾�����̬����Dȼ��ʱ��������Ȳ�泣�������ӻ��и�����������ʵ���A��B��������ˮ��ֻ����ǡ����ȫ��Ӧ��ͼ�з�Ӧ����(��������)������ȥ��

(1)д��B�ĵ���ʽ___________��D�Ľṹ��ʽ___________��
(2)д��A��B��Ӧ�Ļ�ѧ����ʽ____________________________________��
(3)��I����ͨ�����б���C�����NaCl��Һ���Ǻ����Ƽ��һ���ؼ���Ӧ���÷�Ӧ�Ļ�ѧ����ʽΪ___________________________________��
(4)д�����з�Ӧ�����ӷ���ʽ��
����A��Һ�м���M_________________________________��
����A��Һ�м������NaOH��Һ��������________________________��
����֪Fe3O4������M��Һ������F���壬�����ӷ���ʽΪ_______________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012���㽭ʡ��һ�и�����ѧ�����п��Ի�ѧ�Ծ� ���ͣ������
��10�֣���֪AΪ��ʽ�Σ�BΪij�����������Ԫ�صĻ�������³�ѹ��C��D��F��G��I������̬�������ʵ���A��B��������ˮ��ֻ����ǡ����ȫ��Ӧ��ͼ�з�Ӧ�������������⣩������ȥ����ش��������⣺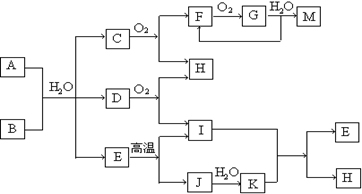
��1��д��B�ĵ���ʽ ��
��2��д��A��B��Ӧ�Ļ�ѧ����ʽ ��
��3��������� G���м�ѹ������������������ ��
G���м�ѹ������������������ ��
��4��д�����з�Ӧ�����ӷ���ʽ��
����A��Һ�м���M ��
����A��Һ�м������NaOH ��Һ�������� ��
��Һ�������� ��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com