
��11�֣���ijͬѧ����ˮ�ʼ��վ����480 mL 0.5 mol/LNaOH��Һ�Ա�ʹ��.
��1����ͬѧӦѡ����������mL������ƿ.
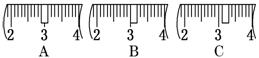
��2���������������ͼ��ʾ�����ͼ����Ӧ����ͼ�е�������������ѡ����ĸ��֮��.
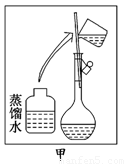

A������ۡ� B������ڡ� C�������
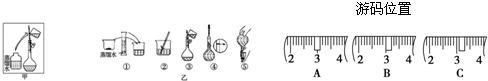
��3����ͬѧӦ��ȡNaOH������������ g��������Ϊ23.1 g���ձ�����������ƽ�ϳ�ȡ����NaOH����ʱ�����ڸ�����ѡȡ����������С��������������ĸ����������ͼ��ѡ������ȷ��ʾ����λ�õ�ѡ����������������ĸ��.
������������
|
|
a |
b |
c |
d |
e |
|
�����С/g |
100 |
50 |
20 |
10 |
5 |
������ͼ��ҽԺ������Һʱ�õ�һƿ��������Ϊ5%��������ע��Һ��ǩ��������۲��ǩ�ϵ��������ݺ���д��
��1������Һ�к�ˮ�������� g.
��2������Һ���ܶ�Ϊ�������� g/mL.
��3������Һ�����ʵ���Ũ��Ϊ��������mol/L
 ȫ�ſ��䵥Ԫ�����������ܸ�ϰϵ�д�
ȫ�ſ��䵥Ԫ�����������ܸ�ϰϵ�д� Ʒѧ˫�ž�ϵ�д�
Ʒѧ˫�ž�ϵ�д� Сѧ��ĩ���100��ϵ�д�
Сѧ��ĩ���100��ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
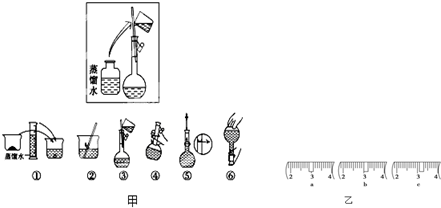
| a | b | c | d | e | |
| �����С/g | 100 | 50 | 20 | 10 | 5 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| a | b | c | d | e | |
| ������/g | 100 | 50 | 20 | 10 | 5 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013�콭��ʡ�˻��а��Ÿ���ѧ����9��˫�ݼ�⻯ѧ�Ծ����������� ���ͣ�ʵ����
(14��)ijͬѧ����ˮ�ʼ��վ����960mL 1 mol��L��1NaOH��Һ�Ա�ʹ�á�
(1)��ͬѧӦѡ��________mL������ƿ��
(2)�������������ͼ��ʾ������ͼ����Ӧ����ͼ�е�________(��ѡ����ĸ)֮�䡣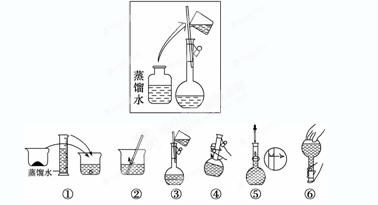
A������ۡ�������B������ڡ�������C�������
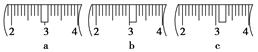
(3)��ͬѧӦ��ȡNaOH����________g��������Ϊ23.1 g���ձ�����������ƽ�ϳ�ȡ����NaOH����ʱ�����ڸ�����ѡȡ����������С________(����ĸ)��������ͼ��ѡ������ȷ��ʾ����λ�õ�ѡ��________(����ĸ)��
������������
| | a | b | c | d | e |
| �����С/g | 100 | 50 | 20 | 10 | 5 |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com