
���ž��÷�չ����������ˮƽ����ߣ���������ʡ����ӵ�����ʽϿ��������ơ�
�� �ұ��� ���������Ժö����������������Ŵ��ܷ⽺������ҵ�����ұ��ĵ����������������������� ��
���������Ժö����������������Ŵ��ܷ⽺������ҵ�����ұ��ĵ����������������������� ��
(2) �ڷ���������Ԫ��ת�������β�����а�װһ����Ϊ��̽�����ĵ绯ѧԪ�����ɲⶨ�ˣ���=��ڴ��Ŀ�����/��ȫȼ����Ҫ�Ŀ�������ֵ������ͼ��ʾ��

�������ٶ�����ʱ����ڴ��Ŀ���������ʱ�ų�β���е���Ҫ��Ⱦ������������ ����ת������ǰ�벿��һ����̼�͵������NOx��ͨ������������Ӧת��Ϊ����Ⱦ�����壬�䷴Ӧ�Ļ�ѧ����ʽΪ������������������������ ��
��3���ⶨ����β����NO��NO2�ķ���֮һ����3����H2O2��Һ��������HNO3������NaOH����Һ�ζ�����Ҫȷ��β����NO��NO2��������ܺͣ������������___________(ѡ�����)��
A��������Ʒ�����������������������
B��NaOH����Һ��Ũ�ȼ������ĵ����
C���ӵζ���ʼ��ָʾ����ɫ�����ʱ�䡡����
D�����ֵ�������������
(4)Ϊ�˽�������β���Դ�������Ⱦ���йز������ü״������Ϊ��������ȼ�ϡ�д���úϳ���(CO��H2)�����״��Ļ�ѧ����ʽ__________________________���ø÷����ϳɼ״�������ŵ���______________________________________________����֪�״�ȼ����Ϊ726kJ/mol����д���״�ȼ�յ��Ȼ�ѧ����ʽ������������ ��������

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
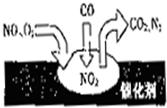 ��2010?�ij�һģ�����ž��÷�չ��������Խ��Խ�࣬ͬʱҲ�����˽�ͨӵ���Ϳ�����Ⱦ������β��װ��������ڴ�������������������õĹ�����ͼ��ʾ������˵����ȷ���ǣ�������
��2010?�ij�һģ�����ž��÷�չ��������Խ��Խ�࣬ͬʱҲ�����˽�ͨӵ���Ϳ�����Ⱦ������β��װ��������ڴ�������������������õĹ�����ͼ��ʾ������˵����ȷ���ǣ��������鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ���������Ժö����������������Ŵ��ܷ⽺������ҵ�����ұ��ĵ�����
���������Ժö����������������Ŵ��ܷ⽺������ҵ�����ұ��ĵ�����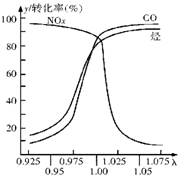
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ���ij�һģ ���ͣ��ʴ���
| A����Ӧ��NOΪ��������N2Ϊ�������� | ||
| B������β������Ҫ��Ⱦ�ɷְ���CO��NO | ||
| C��NO��O2�����ڴ���������ܷ�Ӧ | ||
D����ת���ܷ�ӦΪNO+O2+CO2
|
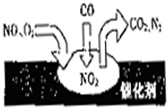
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2010��ɽ��ʡ�ij��и߿���ѧһģ�Ծ��������棩 ���ͣ�ѡ����

 CO+N2
CO+N2�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com