
CH3CH2OH��CH3COOH��NaOH������ѧ��ѧ�ij����Լ�����ش��������⣺
�Ţ�����ͼ��������Ϊͬ���칹�壬��ǰ�ߵ��۵���ں��ߡ�ԭ���� ��
������֬��������Ӧ�У������Ҵ��ܼӿ췴Ӧ�����ʡ�ԭ���� ��
�۽��ڿ��������ճɺ��ȵ�ͭ˿������ˮ�Ҵ��У�ͭ˿������Ϊ��ɫ�����û�ѧ����ʽ��ʾͭ˿����ԭ�� ��
����0��1mol/LNaOH��Һ�ⶨCH3COOH�����ʵ���Ũ�ȣ���ʽ�ζ����ñ���Һ��ϴ�������Һ��������Ҫ���������еζ���
�ٴ˴��ġ���Ҫ��������ָ ��
��ѡ�õ�ָʾ���� ��
�۵ε��յ�ʱ��Һ������Ũ���ɴ�С��˳���� ��
����ˮ�����ƺ�NaOH�����ϼ���ʱ������CH4����Ӧ�Ļ�ѧ����ʽ�� �����ô˷�Ӧ�Ʊ�CH4��װ�����Ʊ� ��ͬ��
���Ҵ���������Ũ��������·�Ӧ���ɾ�����ζ�����ʣ���Ӧ�Ļ�ѧ����ʽ�� ����Ӧ������ ����Ũ����������������� �����⣨���������ɣ���
�Ţ�������Ӽ����γ����
���Ҵ�������ˮ����֬�������Ҵ����Ӷ���������Һ�ĽӴ��档
��CH3CH2OH+CuO CH3CHO+Cu+H2O ��1+1+2�֣�
�Ƣٳ�ȥ�ζ��ܼ��첿�ֵ����ݣ�ʹ�ζ��ܼ��첿�ֳ�����Һ������Һ��߶ȵ���0����0���̶�����
�ڷ�̪
��c��Na+����c��CH3COO-����c��OH-����c��H+�� ��2+1+2��
��CH3COONa+NaOH Na2CO3+CH4�� NH3��O2 ��2+1��
Na2CO3+CH4�� NH3��O2 ��2+1��
��CH3COOH+CH3CH2OH  CH3COOCH2CH3+H2O ������Ӧ
CH3COOCH2CH3+H2O ������Ӧ
��������Ӧ������SO2���ж����� ��2+1+2��
����



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

| �� |
| �� |
| ŨH2SO4 |
| 170�� |
| ŨH2SO4 |
| 170�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
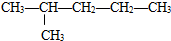 ��
��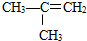
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com