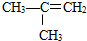����⣺��1����������Ӽ����γ���������Ӽ�����Ĵ��ڵ���ʹ���ʵ��۷е����ߣ���������ȼ���������۵�ߣ�
�ʴ�Ϊ��������Ӽ����γ������
���Ҵ�����ˮ���ܣ��ٸ�����������ԭ���л������������л��ܼ������������������ܼ����Ҵ�����֬�����л��������֬�������Ҵ����Ӷ���������Һ�ĽӴ��棬�ӿ췴Ӧ�����ʣ���
�ʴ�Ϊ���Ҵ�������ˮ����֬�������Ҵ����Ӷ���������Һ�ĽӴ��森
���ڼ��������£�ͭ�ڿ������ܺ�������Ӧ��������ͭ������ͭ���Ҵ��ܷ���������ԭ��Ӧ������ȩ��ˮ��ͭ��CH
3CH
2OH+CuO
CH
3CHO+Cu+H
2O��
�ʴ�Ϊ��CH
3CH
2OH+CuO
CH
3CHO+Cu+H
2O��
��2���ٵζ��ܼ��첿�����ײ������ݣ�����������Һ��Ũ�Ȳ��������Ա����ȥ�ζ��ܼ��첿�ֵ����ݣ�ʹ�ζ��ܼ��첿�ֳ�����Һ��
�ζ��ܵġ�0���̶����Ϸ��������Һ������ڡ�0���̶��Ϸ���Ҳ����������Һ��Ũ�Ȳ���������Ҫ����Һ��߶ȵ���0����0���̶����£�
�ʴ�Ϊ����ȥ�ζ��ܼ��첿�ֵ����ݣ�ʹ�ζ��ܼ��첿�ֳ�����Һ������Һ��߶ȵ���0����0���̶����£�
��ǿ����������ζ�ʱ��Ӧѡ�ü��ȣ�
ǿ����������ζ�ʱ��Ӧѡ�÷�̪��
ǿ����ǿ����ζ�ʱ���ȿ�ѡ�ü��ȣ�Ҳ��ѡ�÷�̪��ָʾ����
��ѡ��̪��
�ʴ�Ϊ����̪��
�۵ε��յ�ʱ���������Ǵ����ƣ���������ǿ�������Σ���ˮ���ʹ��Һ�ʼ��ԣ�������Һ�����������ӵ�Ũ�ȴ���������Ũ�ȣ���������ˮ��Һ����Ȼ��ˮ�⣬�������Ե���Ϊ����������Һ��������Ũ�ȡ����������ԶԶ�������������ӵ�Ũ�ȣ���Ϊ���������ˮ�⣬����������Ũ�ȴ��ڴ��������Ũ�ȣ������⼸������Ũ�ȵĴ�С˳��Ϊ��
c��Na+����c��CH3COO-����c��OH-����c��H+����
�ʴ�Ϊ��c��Na+����c��CH3COO-����c��OH-����c��H+����
��3����ˮ�����ƺ�NaOH�����ϼ���ʱ������CH
4��̼���ƣ�CH
3COONa+NaOH
Na
2CO
3+CH
4���������ƺ��������ƶ��ǹ��壬�ҷ�Ӧ�����Ǽ��ȣ�������NH
3��O
2 ���Ʊ�װ����ͬ��
�ʴ�Ϊ��CH
3COONa+NaOH
Na
2CO
3+CH
4���� NH
3��O
2��
��4���ڼ��������£��Ҵ���������Ũ��������·�Ӧ��������������ˮ��CH
3COOH+CH
3CH
2OH
CH
3COOCH
2CH
3+H
2O���÷�Ӧ��������Ӧ��Ũ������ǿ�����ԣ��������ܱ���ԭ�ɶ���������������ʣ�
�ʴ�Ϊ��CH
3COOH+CH
3CH
2OH
CH
3COOCH
2CH
3+H
2O�� ������Ӧ�� ��������Ӧ������SO
2���ж����壮





 ��
��