
�����й��л����˵����
�ٽṹƬ��Ϊ����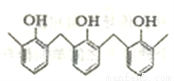 �����ĸ߾���䵥���Ǽ�ȩ�ͱ���
�����ĸ߾���䵥���Ǽ�ȩ�ͱ���
�ڱ�״���£�22.4LHF����������ԼΪ10NA
�ۼ״�ͨ����һ���ж������壬������ֻ�����Լ�
�ܱ��Ӻ�̼������Һ�ķ�Ӧ��
�� ������Ϊ��2,4,4��-����-1-��ϩ
������Ϊ��2,4,4��-����-1-��ϩ
��3-��-1-��ϩ�е�����̼ԭ�Ӳ����ܴ���ͬһƽ��
�ߵ����ʵ����ı��뱽������ȫȼ�������������������
������ȷ���� ����
A. 4 B. 5 C. 6 D. 7
 ��ĩ100�ִ��غ�������ϵ�д�
��ĩ100�ִ��غ�������ϵ�д� Сѧ�������Ծ�ϵ�д�
Сѧ�������Ծ�ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ��2017�����ʡ����2�·�ģ���������ۻ�ѧ�Ծ��������棩 ���ͣ��ƶ���
��ҵ����1,3-����ϩ����ϩ����Ȳ��Ϊԭ�Ϻϳ� ������ͼ���£�
������ͼ���£�


��1����Ӧ�ٵķ�Ӧ������________��B�к��еĹ����ŵ�����__________��
��2��д����Ӧ�ڵĻ�ѧ����ʽ___________��
��3��д��C�Ľṹ��ʽ_________��
��4��д�� ������Ԫ���Һ˴Ź���������4����ͬ���칹��Ľṹ��ʽ________��
������Ԫ���Һ˴Ź���������4����ͬ���칹��Ľṹ��ʽ________��
��5�������Ϻϳ���Ϣ������ϩΪԭ�Ϻϳ�1,6-�Ҷ����������Լ���ѡ��д���ϳɵ�����ͼ______��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2016-2017ѧ�꽭��ʡ�γ��и߶�ѧҵˮƽģ�⣨һ����ѧ�Ծ��������棩 ���ͣ�ѡ����
�������������������(����)
A. ��̿��ˮú�� B. Ũ�����ϡ��
C. ����кͷ�Ӧ D. þ����������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2017�����ʡ�Ƹ��и����������������ۻ�ѧ�Ծ��������棩 ���ͣ�ѡ����
�������в������������ó��Ľ�����ȷ����
��� | ���������� | ���� |
A | ���ؾ�ʯ�����ڱ���̼������Һ�У�һ��ʱ�������ܽ� | Ksp(BaCO3)��Ksp(BaSO4) |
B | ��������ͨ����ˮ�У���Һ��ɫ��ȥ | �����������Ư���� |
C | ��NaOH��NaNO3�Ļ����Һ�м������۲����ȣ���ʪ��ĺ�ɫʯ����ֽ���ڹܿڣ���ֽ���� | NO3����ԭΪNH3 |
D | ��1.0mol��L��1Na2S��Һϡ�͵�0.10mol��L��1�����pH��С | ϡ�ͺ�S2����ˮ��̶ȼ�С |
A. A B. B C. C D. D
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2017�����ʡ���������������и�����һ���������ۻ�ѧ�Ծ��������棩 ���ͣ������
Ϊ��Ч�������������ػ�����ȡ��ʩ���ƴ�����������Ч���ƿ����е������̼����������������Ե���Ϊ��Ҫ��
(1)�������������ڰ��մ�ת�������ɽ�����β������Ҫ��Ⱦ��ת��Ϊ���Ĵ���ѭ�����ʡ�
��֪���� N2(g)��O2(g)��2NO(g) ��H��+180.5kJ��mol-1
��C��CO��ȼ����(��H)�ֱ�Ϊ-393.5kJ��mol-1��-283kJ��mol-1
��2NO(g)��2CO(g)��N2(g)��2CO2(g)) ��H��_______kJ��mol-1
(2)��0.20molNO��0.10molCO����һ���ݻ�Ϊ1L���ܱ������У���Ӧ����������Ũ�ȱ仯��ͼ��ʾ��
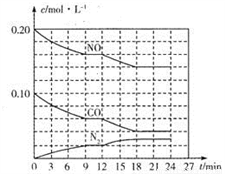
��CO��0-9min�ڵ�ƽ����Ӧ����v(CO)=________mol��L-1��min-1 (������λ��Ч���֣�����12 minʱ�ı�ķ�Ӧ��������Ϊ________��
A.�����¶� B.����NO C.�Ӵ��� D.�����¶�
�ڸ÷�Ӧ�ڵ�18 minʱ�ﵽƽ��״̬��CO2���������Ϊ________��������λ��Ч���֣�����ѧƽ�ⳣ��K=________��������λ��Ч���֣���
(3)ͨ���˹���������ܽ�ˮ��ȼú������CO2ת����HCOOH��O2����֪������0.1mol��L-2��
HCOONa��ҺpH=10����HCOOH�ĵ��볣��Ka=______________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2017�찲��ʡ�����и�����һ��ģ�⿼�����ۻ�ѧ�Ծ��������棩 ���ͣ������
��1��д����̬��ԭ�ӵĵ����Ų�ʽ ��
��2����֪����ˮ�Ȼ�����178�����������������ǵϵ�˫���ڣ�Al2Cl6�����ṹ��ͼ
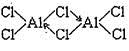
��˫����Al2Cl6��Alԭ�ӵĹ���ӻ������� ��
��3��Bԭ�ӵĵ����� ����ͬ���ܼ����������۵�Ϊ2300�棬����Ϊ ���塣
��4������BP����һ���м�ֵ����ĥӲͿ����ϣ�����ͨ���ڸ���������Χ�£���750�棩���廯������廯��Ӧ�Ƶá�BP������ͼ��ʾ��
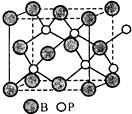
�ٻ������廯������廯�Ŀռ�ṹʽ��
���廯�� ���廯��
����BP������B�Ķѻ���ʽΪ ��
�ۼ��㵱�����������Ϊa pm����ͼ���������ÿ���߳�Ϊ a pm��ʱ����������ԭ�Ӻ���ԭ��֮���������� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2017�찲��ʡ�����и�����һ��ģ�⿼�����ۻ�ѧ�Ծ��������棩 ���ͣ�ѡ����
�����й�ʵ������ͽ��۾���ȷ����
ѡ�� | ���� | ���� |
A | ����Fe(NO3)2��Һʱ�������������� | ����Fe2+ˮ�� |
B | Ũ��������Ƿ�Ӧ����������ͨ��������KMnO4��Һ������ȫ������������Һ�Ϻ�ɫ��ȥ | �������ʵ�顱������������л�ԭ�� |
C | ����Һ�еμ�NaOH��Һ����ʪ���ɫʯ����ֽ�����Թܿ���ֽ������ | ԭ��Һ����NH4+ |
D | ��ʹ�ú�NaF�����࣬����ʹ�����ϵ�Ca5(PO4)3OHת��ΪCa5(PO4)3F����ֹ���� | Ksp[Ca5(PO4)3F]<Ksp[Ca5(PO4)3OH] |
A. A B. B C. C D. D
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2017�콭��ʡ�����и�����һ��ģ�⿼�Ի�ѧ�Ծ��������棩 ���ͣ�ѡ����
�������ӷ���ʽ��ȷ����
A. Fe3O4��������ϡ���Fe3O4��8H+=Fe2++2Fe3++4H2O
B. 4mol/LNaAlO2��Һ��7mol/L����������ϣ�4AlO2-+7H++H2O=3Al(OH)3����A13+
C. 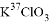 ��Ũ�����ڼ�������������������
��Ũ�����ڼ�������������������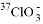 ��6Cl-��6H+=
��6Cl-��6H+= ʮ3Cl2��+3H2O
ʮ3Cl2��+3H2O
D. ��������Һ������ȩ�е�ȩ����CH3CHO+2[Ag��NH3��2]++2OH- CH3COO-+NH4++3NH3+2Ag��+H2O
CH3COO-+NH4++3NH3+2Ag��+H2O
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2016-2017ѧ��ɽ��ʡ�����и�һ��ѧ�ڿ�ѧ���Ի�ѧ�Ծ��������棩 ���ͣ�ѡ����
�������з�Ӧ�ж�����������������ǿ������˳����ȷ���� ( )
�� Cl2+2KI�T�TI2+2KCl
�� 2FeCl3+2HI�T�TI2+2FeCl2+2HCl
�� 2FeCl2+Cl2�T�T2FeCl3
�� I2+SO2+2H2O�T�T2HI+H2SO4
A. Cl2��I2��Fe3+��SO2
B. Cl2��Fe3+��I2��SO2
C. Fe3+��Cl2��I2��SO2
D. Cl2��Fe3+��SO2��I2
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com