
���й��ڷ�Ӧ�ȵ���������ȷ����
A. ��H��(aq)��OH��(aq)===H2O��l����H����57.3 kJ��mol��1��֪����1 mol CH3COOH����Һ�뺬1 mol NaOH����Һ��ϣ��ų�����Ϊ57.3 kJ
B. ��C(ʯī��s)===C(���ʯ��s)����H��1.9 kJ��mol��1����֪��ʯī�Ƚ��ʯ���ȶ�
C. 500�桢30 MPa�£���0.5 mol N2��1.5 mol H2�����ܱ������г�ַ�Ӧ����NH3(g)������19.3 kJ�����Ȼ�ѧ����ʽΪN2(g)��3H2(g) 2NH3(g) ��H����38.6 kJ��mol��1
2NH3(g) ��H����38.6 kJ��mol��1
D. ����ı�ȼ���ȣ���H��Ϊ��890.3 kJ��mol��1�������ȼ�յ��Ȼ�ѧ����ʽ�ɱ�ʾΪCH4(g)��2O2(g)===CO2(g)��2H2O(g)����H����890.3 kJ��mol��1
 Сѧ��ʱ��ѵϵ�д�
Сѧ��ʱ��ѵϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ��2016~2017ѧ�꽭��ʡ��Ǩ�и߶�ѧҵˮƽ����ģ�⣨������ѧ�Ծ��������棩 ���ͣ�ѡ����
��ҵ�������������մɷ�ӦΪ3SiCl4��2N2��6H2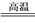 Si3N4��12HCl���йظ÷�Ӧ˵����ȷ����
Si3N4��12HCl���йظ÷�Ӧ˵����ȷ����
A. SiCl4�������� B. N2ʧ���� C. H2����ԭ D. N2������ԭ��Ӧ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2016-2017ѧ��㶫ʡտ���и߶���ѧ����ĩ���п��Ի�ѧ�Ծ��������棩 ���ͣ�ѡ����
���и�����Ͷ��ˮ�У���ٽ�ˮ�ĵ����ʹ��Һ�����Ե���
A. NaHSO4 B. Na3PO4 C. CH3COOH D. NH4Cl
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2016-2017ѧ�긣��ʡ�����и߶���ѧ����ĩ���Ի�ѧ�Ծ��������棩 ���ͣ�ѡ����
�ڳ����£���pH��9��NaOH��Һ��pH��11��NaOH��Һ�������Ϻ���Һ��pH��ӽ���
A. 9.3 B. 9.7 C. 10 D. 10.7
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2016-2017ѧ�긣��ʡ�����и߶���ѧ����ĩ���Ի�ѧ�Ծ��������棩 ���ͣ�ѡ����
��������ָ������������½���ѧ��ת��Ϊ���ܵ�װ�ã��乤��ԭ����ͼ��ʾ�������й������ص�˵���������
A. ������Ӧ����CO2����
B. ����ٽ��˷�Ӧ�е��ӵ�ת��
C. ����ͨ������Ĥ�Ӹ���������������
D. ����ܷ�ӦΪC6H12O6+6O2=6CO2+6H2O
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2016-2017ѧ��ӱ�ʡ�żҿ��и߶���ѧ����ĩ���Ի�ѧ�Ծ��������棩 ���ͣ������
�������£���һԪ��HA����Һ��KOH��Һ�������ϣ����Ի�Ϻ���Һ������仯����ʵ���������±���
ʵ����� | ��ʼŨ�ȣ���mol��L��1�� | ��Ӧ����Һ��pH | |
c��HA�� | c��KOH�� | ||
�� | 0.1 | 0.1 | 9 |
�� | x | 0.2 | 7 |
��ش�
��1��HA��Һ��KOH��Һ��Ӧ�����ӷ���ʽΪ________��
��2��ʵ��ٷ�Ӧ�����Һ����ˮ�������c��OH������________mol��L��1��x________0.2mol��L��1���������������������
��3�����й���ʵ��ڷ�Ӧ�����Һ˵������ȷ����________������ĸ����
a����Һ��ֻ����������ƽ��
b����Һ�У�c��A������c��HA����0.1mol��L��1
c����Һ�У�c��K������c��A������c��OH������c��H����
����֪2H2��g����O2��g����2H2O��1�� ��H����572kJ��mol��1��ij����ȼ�ϵ�������ɶ��ʯī��Ϊ�缫��KOH��ҺΪ�������Һ��
��4��д���õ�ع���ʱ�����ĵ缫��Ӧʽ________��
��5����������ȼ�ϵ��ÿ�ͷ�228.8kJ����ʱ��������1molҺ̬ˮ����õ�ص�����ת����Ϊ________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2016-2017ѧ��ӱ�ʡ�żҿ��и߶���ѧ����ĩ���Ի�ѧ�Ծ��������棩 ���ͣ�ѡ����
��֪�¶�Tʱˮ�����ӻ�����ΪKW�����¶��£���Ũ��Ϊamol��L��1��һԪ��HA��bmol��L��1��һԪ��BOH�������ϣ����ж�����Һ�����Ե�������
A. a��b
B. �����Һ��pH��7
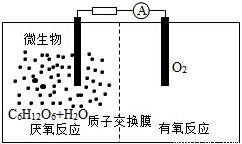
C. �����Һ��c(H+)= mol?L-1
mol?L-1
D. �����Һ�У�c��H������c��B������c��OH������c��A����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2016-2017ѧ��ӱ�ʡ�żҿ��и�һ��ѧ����ĩ���Ի�ѧ�Ծ��������棩 ���ͣ������
��ҵ���ó�����Fe2O3��FeO��Ҳ����Al2O3��MnO2��CuO��SiO2�ȣ��Ʊ��̷���FeSO4��7H2O�����������£�
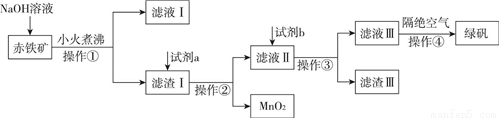
��1����Һ������������������________��
��2���Լ�aΪ________���Լ�bͶ�뵽��Һ���������з�Ӧ�Ļ�ѧ����ʽΪ________��
��3��ʵ�����в���������IJ���������________��
��4�������ܵ�����Ϊ________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2016-2017ѧ������ʡ�����и߶���ѧ����ĩ���ԣ��ģ���ѧ�Ծ��������棩 ���ͣ�ѡ����
��ʵ�����У������¹ʴ�����ʵ�������ȷ����
A. �д���������й©ʱ�����ռ���Һ��ʪ�������棬��Ѹ���뿪�ֳ�
B. �������Ż�ȼ��ʱ������ĭ��������
C. ����Ũ����մ��Ƥ���ϣ�����������������Һ��ϴ
D. ����ʱ����������������ֽ��һ�߽Ӵ�
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com