
��һ���¶��£�������X��Y��1.6mol����10L�����ܱ������У��������·�Ӧ��
X��g��+ Y��g�� 2Z��g��,�ڷ�Ӧ�����У�X�����ʵ�����ʱ��Ĺ�ϵ���±���ʾ��
2Z��g��,�ڷ�Ӧ�����У�X�����ʵ�����ʱ��Ĺ�ϵ���±���ʾ��
t/min | 0 | 2 | 4 | 6 | 8 | ���� | 16 | 18 |
n(X)/mol | 1.600 | 1.200 | 1.100 | 1.075 | ���� | ���� | 1.000 | 1.000 |
����˵������ȷ����( )
A. 4��6minʱ�����Z��ƽ����Ӧ����Ϊ2.5��10-3mol/(L��min)
B. ���¶��¸÷�Ӧ��ƽ�ⳣ��K=1.44
C. ��ƽ��������¶ȣ� ��С��������Ӧ
��С��������Ӧ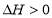
D. �����������������䣬�ٳ���1.6mol Z������ƽ���Z�������������
 ����ѧ��ţ��Ӣ��ϵ�д�
����ѧ��ţ��Ӣ��ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ��2016-2017ѧ��㶫ʡ�����и�һ��ѧ������ĩ���Ի�ѧ�Ծ��������棩 ���ͣ��ƶ���
����ͼ��ʾ�ķ�Ӧ��ϵ�У����ֲ��ﱻ��ȥ����֪��ɫ����X�ķֽ������ֻ��A�ǰ�ɫ���壬X��A��E��G����ɫ��Ӧ��Ϊ��ɫ��

��ش��������⣺
(1)д��X��A+B+C�Ļ�ѧ����ʽ��_____________________
(2)д��E��G�Ļ�ѧ����ʽ��_________________
(3)д��G��C��Ӧ�����ӷ���ʽ��_____________________
(4)д������Һ��X+E��A��Ӧ�����ӷ���ʽ��_________________
�������п�ͼ�ش����⣨����ʱ������ʽ�е�M��E������Ӧ��Ԫ�ط��ű�ʾ����

��ش��������⣺
��5��д��M����ϡH2SO4��H2O2���Һ�Ļ�ѧ����ʽ��________________��
��6��ijͬѧȡY����Һ������г�ȥH2O2��,��ȴ,�ټ������KI��Һ����Ϊ��ɫ��д���������KI��Һ�ķ�Ӧ�����ӷ���ʽ��_____________________��
��7��д��Cl2��Z����ΪK2EO4�Ļ�ѧ����ʽ��______________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2017�콭��ʡ������ѧ���ڳ����Ի�ѧ�Ծ��������棩 ���ͣ������
CO2�Ļ������öԼ������������ŷš������������滷��������Ҫ���塣����CO2��CH4�������ƺϳ���(��Ҫ�ɷ�CO��H2)�����������в��ַ�Ӧ���Ȼ�ѧ����ʽΪ�� ��CH4(g) = C(s) + 2H2(g) ��H = +75.0 kJ��mol-1
��CO2(g) + H2(g) = CO(g) + H2O(g) ��H = +41.0 kJ��mol-1
��CO(g) + H2(g) = C(s) + H2O(g) ��H = -131.0 kJ��mol-1
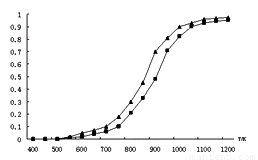
��1����ӦCO2(g) + CH4(g) = 2CO(g) + 2H2(g)�ġ�H= ___________��
��2���̶�n(CO2)= n(CH4)���ı䷴Ӧ�¶ȣ�CO2��CH4��ƽ��ת���ʼ���ͼ��
��ͬ�¶���CO2��ƽ��ת���� ____________(����ڡ���С�ڡ�)��CH4��ƽ��ת���ʣ���ԭ���� __________________________��
�ڸ����½��и÷�Ӧʱ������Ӧ�����ɡ���̼��(̼����)����ɴ����ж��������·�Ӧ�����Է����е�ԭ����_________________��
��3��һ��������Pd-Mg/SiO2������ʹCO2�����黯���Ӷ����Ϊ�����䷴Ӧ������ͼ��ʾ���÷�Ӧ�Ļ�ѧ����ʽΪ_____________________����Ӧ������̼Ԫ�صĻ��ϼ�Ϊ-2�۵��м�����__________��

��4��±ˮ���������̵�����CO2��ͬʱ��������ʵ���Է��ηϣ������漰��һ����Ӧ��CaSO4 + Na2CO3 == CaCO3 + Na2SO4����ﵽƽ�����Һ��c(CO32-)/c(SO42-) = __________������Ksp(CaSO4)��Ksp(CaCO3)��ʾ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2017�콭��ʡ������ѧ���ڳ����Ի�ѧ�Ծ��������棩 ���ͣ�ѡ����
�����й����ʵ�������Ӧ�����Ӧ���ǣ�������
A. ̼������Һ�ʼ��ԣ�������ϴȥ��м���������
B. �������ۻ������������ɻ�������Ľṹ����
C. ̿���л�ԭ�ԣ�������ұ���ơ�þ�����Ƚ���
D. Ũ�������ǿ�����ԣ������ڸ��������������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2016-2017ѧ�����ʡ��ɳ�и߶���ѧ�ڵ�һ��ģ���⻯ѧ�Ծ��������棩 ���ͣ�ѡ����
��ϩ�����ת����ϵ��ͼ������˵����ȷ����

A. ����ϩ�Ǵ����� B. CH3 OCH3���Ϊͬ���칹��
C. XΪC12 D. �ס��ҷ�Ӧ����Ϊȡ����Ӧ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2016-2017ѧ�����ʡ��ɳ�и߶���ѧ�ڵ�һ��ģ���⻯ѧ�Ծ��������棩 ���ͣ�ѡ����
��2A��B 3C��4D��Ӧ�У���ʾ�÷�Ӧ����������
3C��4D��Ӧ�У���ʾ�÷�Ӧ����������
A. �ԣ�A���� 0.5 mol/��L���� B. �ԣ�B���� 0.3 mol/��L����
C. �ԣ�C���� 12 mol/��L��min�� D. �ԣ�D���� 6 mol/��L��min��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2016-2017ѧ�꽭��ʡ�ϲ��и�һ��ѧ�ڷְ�Ի�ѧ�Ծ��������棩 ���ͣ������
��һ����Һ�����ܺ��д�����Mg2����Fe3����Al3����Cu2����Na����H����SO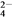 ��CO
��CO �е�һ�ֻ��֣�ȡ����Һ������ʵ�飺
�е�һ�ֻ��֣�ȡ����Һ������ʵ�飺
��ȡ������Һ�����������ữ��BaCl2��Һ���а�ɫ�������ɣ�
��ȡ������Һ����������Ʒ�ĩ����Һ���а�ɫ�����������ݳ���ɫ��ζ�����壬����Na2O2�����ʵ������������������ʵ�����ͼ��ʾ�����ƶϣ�

(1)��Һ��һ�����е�������______________��
(2)��Һ�п϶������е�������________________��
(3)���ܺ��е�������____________,��Ҫȷ�������ӵĴ�����Ҫ�õ���ʵ�鷽����____________
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2016-2017ѧ�꽭��ʡ�ϲ��и�һ��ѧ�ڷְ�Ի�ѧ�Ծ��������棩 ���ͣ�ѡ����
�����ײ��ϡ��ǵ�����Ͽ�ѧ�о���ǰ�أ�1����(nm)=10-9m�����о��ɹ��㷺Ӧ���ڴ������¿�ѧ�У������ײ��ϡ���ָ�о�����������ֱ���Ӽ���������ʮ���IJ��ϣ� �罫�����ײ��ϡ���ɢ��Һ���ɢ���У����û������ܾ��е������ǣ� ��
A. �ж����ЧӦ B. ��ȫ������Ĥ
C. ��������ֽ D. ���÷�ɢϵ���ȶ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2016-2017ѧ�������ʡĵ�����и߶���ѧ�ڿ�ѧ��⻯ѧ�Ծ��������棩 ���ͣ�ѡ����
�����������Һ��ϡ���ᷴӦ�Ļ�ѧ����ʽΪ��
Na2S2O3 + H2SO4 = Na2SO4 + SO2 + S�� + H2O
���и���ʵ�������ȳ��ֻ��ǵ���
ʵ�� | ��Ӧ�¶�/�� | Na2S2O3��Һ | ϡH2SO4 | H2O | ||
V/mL | c/(mol��L-1) | V/mL | c/(mol��) | V/mL | ||
A | 25 | 5 | 0.1 | 10 | 0.1 | 5 |
B | 25 | 5 | 0.2 | 5 | 0.2 | 10 |
C | 35 | 5 | 0.1 | 10 | 0.1 | 5 |
D | 35 | 5 | 0.2 | 5 | 0.2 | 10 |
A. A B. B C. C D. D
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com