
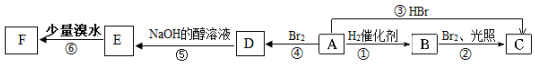
 ���ṹ��ʽ����
���ṹ��ʽ�������� A����ʽΪC6H12����A����̼ԭ�Ӷ���ͬһƽ���ϣ�ӦΪC��CH3��2=C��CH3��2��Ϊ2��3-����-2-��ϩ����ת����ϵ��֪BΪCH��CH3��2CH��CH3��2��CΪCH3CH��CH3��CBr��CH3��2��DΪC��CH3��2BrC��CH3��2Br��EΪCH2=C��CH3��C��CH3��=CH2����������ˮ��Ӧ���ɵ�FΪCH2BrC��CH3��=C��CH3��CH2Br���Դ˽����⣮
��� �⣺��1�������Ϸ�����֪AΪC��CH3��2=C��CH3��2������Ϊ2��3-����-2-��ϩ��A��ͬ���칹����һ�ȴ���ֻ��һ�ֵ��ǻ����飬�ṹ��ʽΪ ��
��
�ʴ�Ϊ��C��CH3��2=C��CH3��2��2��3-����-2-��ϩ�� ��
��
��2����ת����ϵ��֪�٢ۢܢ�Ϊ�ӳɷ�Ӧ����Ϊȡ����Ӧ����Ϊ��ȥ��Ӧ���ʴ�Ϊ���ڣ�
��3�������Ϸ�����֪CΪCH3CH��CH3��CBr��CH3��2���ʴ�Ϊ��CH3CH��CH3��CBr��CH3��2��
��4��D������ȥ��Ӧ����E����D��E�Ļ�ѧ����ʽΪC��CH3��2BrC��CH3��2Br+2NaOH$��_{��}^{�Ҵ�}$CH2=C��CH3��C��CH3��=CH2+2NaBr+2H2O��
�ʴ�Ϊ��C��CH3��2BrC��CH3��2Br+2NaOH$��_{��}^{�Ҵ�}$CH2=C��CH3��C��CH3��=CH2+2NaBr+2H2O��
��5��EΪCH2=C��CH3��C��CH3��=CH2����������ˮ��Ӧ���ɵ�FΪCH2BrC��CH3��=C��CH3��CH2Br������ʽΪCH2=C��CH3��C��CH3��=CH2+Br2��CH2BrC��CH3��=C��CH3��CH2Br��
�ʴ�Ϊ��CH2=C��CH3��C��CH3��=CH2+Br2��CH2BrC��CH3��=C��CH3��CH2Br��
���� ���⿼���л�����ƶϣ�Ϊ��Ƶ���㣬���ؿ���ѧ���ķ���������ע������л���Ľṹ�ص�����ʣ��������ʵ�ת����ϵ�ͷ�Ӧ�������ѶȲ���


 ���Ŀ��ּ�����ҵ�����ҵ����������ϵ�д�
���Ŀ��ּ�����ҵ�����ҵ����������ϵ�д� ����ѵ��ϵ�д�
����ѵ��ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | �� NaAlO2��Һ�еμ� NaHCO3 ��Һ���г������������� | |
| B�� | Na��Mg��Fe �Ƚ�����һ����������ˮ��Ӧ������ H2�Ͷ�Ӧ�ļ� | |
| C�� | ͬ����Ԫ�صļ������ӻ�ԭ��Խǿ��ˮ��̶�Խ�� | |
| D�� | ����ͨʽ CnH2n+2�IJ�ͬ����һ����Ϊͬϵ�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | 0.50mol•L-1 | B�� | 0.75mol•L-1 | C�� | 1.00mol•L-1 | D�� | 3.18mol•L-1 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | A������һ������C | |
| B�� | �÷�ӦΪ���ȷ�Ӧ���ʲ��ؼ��Ⱦ�һ���ܷ��� | |
| C�� | B������һ������D | |
| D�� | A��B��������һ������C��D�������� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ԭ�Ӻ�����10 �����ӵ���ԭ�ӣ�${\;}_{8}^{18}$O | |
| B�� | ������Ľṹʽ��H-Cl-O | |
| C�� | CO2�ı���ģ�ͣ� | |
| D�� | �廯淋ĵ���ʽ��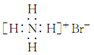 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ��Ȼ���в���������̬��̼ | B�� | ̼�Ļ����������ڶ࣬�ֲ����� | ||
| C�� | ��������ŷŶ�����̼���γ����� | D�� | ú��ʯ�͡���Ȼ�����ڿ�����̼��Դ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ����Na2S��Һ��ͨ��SO2���������ɫ���� | |
| B�� | �������ᶼ�Ƕ�Ԫ�ᣬ�������������Ʒ�Ӧ������ʽ�κ����� | |
| C�� | ���������ˮ��Һ���ÿ����ж������ | |
| D�� | �������ᡢ��������Һ�еμ���ˮ���ᷢ��������ԭ��Ӧ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
| A�� | ��ȩ��������Һ��Ӧ��CH3CHO+2[Ag��NH3��2]OH $\stackrel{��}{��}$CH3COO-+NH4++2Ag��+3NH3+H2O | |
| B�� | ������Һ������������ͭ��Ӧ��2CH3COOH+Cu��OH��2��Cu2++2CH3COO-+2H2O | |
| C�� | ��������Һ��ͨ������������̼��2C6H5O-+CO2+H2O��2C6H5OH+CO32- | |
| D�� | ������ˮ�������Ҵ���CH3CH2Br+OH-$��_{��}^{H_{2}O}$CH3CH2OH+Br- |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����

| A�� | ����ʽΪC10H16O | |
| B�� | ���մ�Ϊ�����廯���� | |
| C�� | ���ϵ�һ��ȡ���������� | |
| D�� | ʹ���Ը��������Һ����ˮ��ɫ��ԭ����ͬ |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com