
��̬��Aȼ�յ��¶��ر�ߣ����������ӽ������и��������A��̼����Ԫ�ص�������Ϊ12:1����֪B����Na������Ӧ��D��E�к����Ȼ�����ش�

(1)�л���B�Ĺ�����Ϊ_________________��
(2)д���л���Cת��ΪB�Ļ�ѧ��Ӧ����ʽ��__________________��
(3)��Ϊ�ӳɷ�Ӧ��д���÷�Ӧ�Ļ�ѧ����ʽ��___________________��
(4)����˵������ȷ����_______��
A��A�����е�����ԭ����ͬһֱ����
B���л���A��ʹ��ˮ��������Ȼ�̼��Һ��ɫ���ҷ�Ӧ������ȫ��ͬ
C����Ӧ��Ϊ������Ӧ����Ӧ��Ϊ��ԭ��Ӧ
D��������B��D���ܱ�H2��ԭΪC
 ��У����ϵ�д�
��У����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ʡ���IJ���ѧ2017�������ѧ��������ѧ�Ծ� ���ͣ������
������(CH3OCH3)����Ϊ21���͵�����ȼ�ϣ���CO��H2Ϊԭ��������������Ҫ��������������Ӧ��
��ѧ��Ӧ����ʽ | ��ѧƽ�ⳣ�� | |
��CO(g)��2H2(g) | ��H1=-99 kJ•mol-1 | K1 |
��2CH3OH(g) | ��H2����24 kJ•mol-1 | K2 |
��CO(g)��H2O(g) | ��H3����41 kJ•mol-1 | K3 |
��1���ù��յ��ܷ�ӦΪ3CO(g)��3H2(g) CH3OCH3(g)��CO2(g) ��H
CH3OCH3(g)��CO2(g) ��H
�÷�Ӧ��H��__________________����ѧƽ�ⳣ��K��____________________(�ú�K1��K2��K3�Ĵ���ʽ��ʾ)��
��2��ij�¶��£���8.0molH2��4.0molCO�����ݻ�Ϊ2L���ܱ������У�������Ӧ��4H2(g)+2CO(g)  CH3OCH3(g)+H2O(g)��10 ���Ӻ�Ӧ��ƽ�⣬��ö����ѵ��������Ϊ25%����CO��ת����Ϊ________��
CH3OCH3(g)+H2O(g)��10 ���Ӻ�Ӧ��ƽ�⣬��ö����ѵ��������Ϊ25%����CO��ת����Ϊ________��
��3�����д�ʩ�У������CH3OCH3���ʵ���________��
A������������� B�������¶� C�����ø�Ч���� D������ѹǿ
��4���ù����з�Ӧ�۵ķ��������CH3OCH3�IJ��ʣ�ԭ����_______________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2016-2017ѧ���Ĵ�ʡ�ɶ��б���У���߶�3���¿���ѧ�Ծ��������棩 ���ͣ�ѡ����
��NO3����Ϊ�ȵ�������ǣ� ��
A. SO32- B. BF3 C. CH4 D. NO2
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2016-2017ѧ��ɽ��ʡ�����и�һ3���¿���ѧ�Ծ��������棩 ���ͣ�ѡ����
Ԫ�ص�ԭ�ӽṹ���������ʺ����ڱ��е�λ�ã�����˵����ȷ���ǣ�������
A. Ԫ��ԭ�ӵ���������������Ԫ�ص�����ϼ�
B. �����ԭ���У�����˽Ͻ����������˶��ĵ��������ϸ�
C. Ԫ�����ڱ���λ�ڽ����ͷǽ����ֽ��߸�����Ԫ�����ڹ���Ԫ��
D. Si��P��S�õ�������������������Ӧˮ��������Ծ�������ǿ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2017���㽭ʡ����������ȫ������3��������ѡ����Ŀ����ѧ�Ծ��������棩 ���ͣ�ʵ����
��Zn����Ҫ����Fe��Al��Pb���ʣ�����������ȡH2�����������Һ�Ʊ�����п����(ZnSO4��7H2O)��Al2O3��Fe2O3���������£�
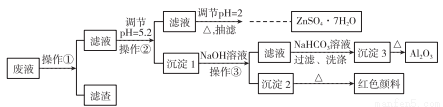
��֪Al3+��Fe3+��Zn2+������������ȫ������pH�ֱ�Ϊ5.2��4.1��8.5��ZnSO4��7H2O����������ˮ���绯���ش��������⣺
(1)����pH=2��Ŀ����______________������pH=2���ɼ���_________���ѧʽ����
(2)д�����ɳ���3�Ļ�ѧ����ʽ��______________________��
(3)����Ũ��ZnSO4��Һ���ּ���ƷĤʱ��Ҫֹͣ���ȵ���Ҫԭ����_____________��
(4)ijͬѧ����ͼ��ʾ��װ�ó��ˡ�
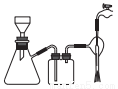
���йس��˵�˵����ȷ����__________��
A.���˵�Ŀ����Ҫ�ǵõ��ϸ���ij���
B����ֽ��ֱ��Ӧ��С��©���ھ������ܸ�סȫ��С��
C��ͼ����һ������
D�����˽�����������ƿ��֧�ܿڵ�����Һ
�ڳ��ˣ�ϴ�ӳ����ľ��������___________________________��
(5)Ϊ�õ������ZnSO4��7H2O��Ʒ��ѡ����﷽����________��
A.���Ⱥ�� B����ŨH2SO4����
C���þƾ�ϴ�� D���ڿ����а�Ȼ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2017���㽭ʡ����������ȫ������3��������ѡ����Ŀ����ѧ�Ծ��������棩 ���ͣ�ѡ����
T�棬��2 mol X��1 mol Y����һ���Ϊ1L�����ܱ������У���֪��2X(g)+Y(g)  2Z(s) ��H=-M kJ��mol-1��10 min��ﵽƽ�⣬����0.2 mol Z�����ų�����N kJ������˵����ȷ���� ( )
2Z(s) ��H=-M kJ��mol-1��10 min��ﵽƽ�⣬����0.2 mol Z�����ų�����N kJ������˵����ȷ���� ( )
A. ��10 minʱ��X�ķ�Ӧ����Ϊ0.02 mol��L-1��min-l
B. ��0��10 min�ڣ�Y�ķ�Ӧ����Ϊ mol��L-1��min-l
mol��L-1��min-l
C. ����Z�����ʵ����ӿ��淴Ӧ����
D. ��Ӧ��ƽ�����T�棬ͨ��ϡ����������ѹǿ����ѧ��Ӧ���ʱ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2017���㽭ʡ����������ȫ������3��������ѡ����Ŀ����ѧ�Ծ��������棩 ���ͣ�ѡ����
����˵������ȷ����
A. �����£�1 mol�����������ȫȡ����Ҫ2 mol����
B. ��һ�������±���Һ�塢Ũ���ᡢŨ����ֱ���ȡ����Ӧ�������屽���������������
C. CH3COOCH2CH3��CH3CH2OOCCH3������ͬ�ķе�
D. CH3CHO��HCOOH��HCOOCH3���ܷ���������Ӧ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2016-2017ѧ��ӱ�ʡ�߶���ѧ��3���¿���ѧ�Ծ��������棩 ���ͣ��ƶ���
��֪�������ڼ��������·������·�Ӧ��RCOOR�䣫NaOH�D��RCOONa��R��OH��ȩ��һ���������ܱ����������ᡣij�����ĺ���������A������Է�������Ϊ88��������C��H��O ��ԭ�Ӹ���֮��Ϊ2��4��1��A����������֮���ת����ϵ���£�

��ش��������⣺
(1)д�� A��E �Ľṹ��ʽ��
A___________________��E_________________��
(2)д�� C �� D ��һ�������·�����Ӧ�Ļ�ѧ����ʽ�� ____________________��
(3)A��ͬ���칹���������������________�֡�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2016-2017ѧ�갲��ʡ�����и�һ��ѧ�ڵ�һ�νμ�⻯ѧ�Ծ��������棩 ���ͣ�ѡ����
ѧ��������ʵ�飺��һ�����ڵ���?KI��Һ�У���������NaClO��Һ������������ϡ���ᣬ��Һ�����������ڶ�������������ɫ��Һ�У��μ�������Na2SO3��Һ����ɫ����ʧ�����������и�ͬѧ��ʵ��ԭ���Ľ��ͺ����ý��۲���ȷ����( )
A�������ԣ�ClO��>I2>SO42��
B����ɫ��ʧ��ԭ����Na2SO3��Һ��Ӧ����SO2����Ư����
C������?KI��Һ��������ΪI����ClO������ΪI2��I2�����۱���
D������Na2SO3��Һ������ˮ����ˮ��ɫ
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com