
��9�֣��л���A��B��C����ͼ��ʾת����ϵ��A�ķ���ʽΪC3H4O2��A����Br2�����Ȼ�̼��Һ�����ӳɷ�Ӧ��1 mol A����1molNaHCO3��Һǡ����ȫ��Ӧ��B����Ԫ��������A��ͬ����Է�������Ϊ46������̼����������Ϊ
52.2���������������Ϊ13�����Իش��������⣺
��1��A�����������ŵ�����Ϊ ��
��2��B�ķ���ʽΪ ��B��ͬϵ��D����Է�������Ϊ60����D���ܵĽṹ��ʽΪ ��
��3��A��B��Ӧ����C�Ļ�ѧ����ʽΪ ��
�÷�Ӧ���� ��Ӧ��
��4��A��B�Ļ���ﹲ1mol�����۶����Ժ��ֱ�����ϣ���ȫȼ��ʱ������ʼ��Ϊ��ֵ���� ��
a. ������������ b. ����ˮ���� c. ���ɶ�����̼����

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
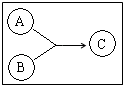 �л���A��B��C����ͼ��ʾת����ϵ��A�ķ���ʽΪC3H4O2��A����Br2�����Ȼ�̼��Һ�����ӳɷ�Ӧ��1mol A����1molNaHCO3��Һǡ����ȫ��Ӧ��B����Ԫ��������A��ͬ����Է�������Ϊ46������̼����������Ϊ52.2%�������������Ϊ13%���Իش��������⣺
�л���A��B��C����ͼ��ʾת����ϵ��A�ķ���ʽΪC3H4O2��A����Br2�����Ȼ�̼��Һ�����ӳɷ�Ӧ��1mol A����1molNaHCO3��Һǡ����ȫ��Ӧ��B����Ԫ��������A��ͬ����Է�������Ϊ46������̼����������Ϊ52.2%�������������Ϊ13%���Իش��������⣺�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
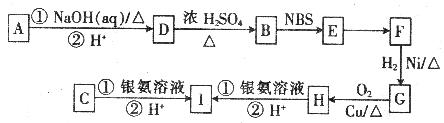
��8�֣��л���A��B��C�ķ���ʽ��ΪC5H8O2���䲿�ֽṹ���������±���
| ��� | A | B | C |
| ���ֽṹ������ | ��Ԫ��״������ | �����к���һ����������ʹ������Ȼ�̼��Һ��ɫ | ̼����֧����1molC����������Ӧʱ����4 mol Ag |
�л���A��B��C����ͼ��ʾ��ת����ϵ��
��֪��
��ش��������⣺
��1��A�Ľṹ��ʽΪ
��2��Dת��ΪB�Ĺ����У����ɵ�һ����ʹ������Ȼ�̼��Һ��ɫ�ĸ�����Ľṹ��ʽΪ
��3��Eת��ΪF�ķ�Ӧ����Ϊ
��4��д��C��������������Һ��Ӧ�Ļ�ѧ����ʽ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2010�꺣��ʡ�����и�����ѧ�ڸ߿����У�һ����ѧ���� ���ͣ������
��9�֣��л���A��B��C����ͼ��ʾת����ϵ��A�ķ���ʽΪC3H4O2��A����Br2�����Ȼ�̼��Һ�����ӳɷ�Ӧ��1 mol A����1molNaHCO3��Һǡ����ȫ��Ӧ��B����Ԫ��������A��ͬ����Է�������Ϊ46������̼����������Ϊ
52.2���������������Ϊ13�����Իش��������⣺
��1��A�����������ŵ�����Ϊ ��
��2��B�ķ���ʽΪ ��B��ͬϵ��D����Է�������Ϊ60����D���ܵĽṹ��ʽΪ ��
��3��A��B��Ӧ����C�Ļ�ѧ����ʽΪ ��
�÷�Ӧ���� ��Ӧ��
��4��A��B�Ļ���ﹲ1mol�����۶����Ժ��ֱ�����ϣ���ȫȼ��ʱ������ʼ��Ϊ��ֵ���� ��
a. ������������ b. ����ˮ���� c. ���ɶ�����̼����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2010�꽭��ʡ����һ�и�����ѧ�ڵڶ��ζο���ѧ���� ���ͣ������
��8�֣��л���A��B��C�ķ���ʽ��ΪC5H8O2���䲿�ֽṹ���������±���
| ��� | A | B | C |
| ���ֽṹ������ | ��Ԫ��״������ | �����к���һ����������ʹ������Ȼ�̼��Һ��ɫ | ̼����֧����1molC�� ��������Ӧʱ����4 mol Ag ��������Ӧʱ����4 mol Ag |
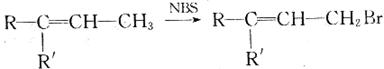
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com