
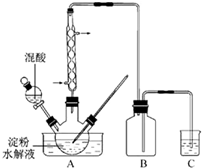
���ᾧ�壨2C2O4��2H2O�������ֽ⣺�ٽ��ֽ������ͨ�����������ձ������ֲ���ˮ�飩����Ȼ�����ɵ�����ͨ�����ʯ��ˮ�У�ʯ��ˮ����ǡ��ס���ͬѧ��Ϊ��
|
| A������֤ʣ�������Ƿ��ȼ��ȼ��ʱ�������ɫ |
| B����ʣ�����廹ԭ�ȵ�CuO��ĩ���۲������ɫ�ı仯 |
| C����ʣ������ͨ����ˮ�У�����ˮ�Ƿ���ɫ |
| D����ʣ����������ȼ�գ�������ˮ����ͭ����ȼ�ղ��� |
 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ��2011?���գ�������һ����Ҫ�Ļ�����Ʒ��ʵ������������������ˮ��Һ�Ʊ������װ����ͼ14��ʾ�����ȡ�����������̶�װ�þ�����ȥ��
��2011?���գ�������һ����Ҫ�Ļ�����Ʒ��ʵ������������������ˮ��Һ�Ʊ������װ����ͼ14��ʾ�����ȡ�����������̶�װ�þ�����ȥ���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013�����ʡ�߶���ѧ�����п��Ի�ѧ�Ծ� ���ͣ�ʵ����
��12�֣����ᾧ�����ɿɱ�ʾΪH 2C2O4��xH2O��Ϊ�ⶨxֵ����������ʵ�飺�ٳ�ȡWg���ᾧ�����100.0 mLˮ��Һ����ȡ25.0 mL���������Һ������ƿ�У���������ϡH2SO4����Ũ��Ϊc mol��L-1��KMnO4��Һ�ζ����ζ�ʱ���������ķ�ӦΪ��

�Իش��������⣺
��1����ƽ������ѧ����ʽ��
��2����ʵ��ζ�ʱ��ָʾ��Ӧ���������� ����ӡ����ӡ�����
��3�����ζ�ʱ����Ӧǰ������ζ����ֱ�Ϊa mL��b mL����ʵ���õ����������Һ�����ʵ���Ũ��Ϊ���������������������� ���ɴ˼�������ᾧ���xֵ���������������� ��
��4�����ζ�ʱ����KMnO4��Һ����ö�����Ũ�ȱ�С�����ɴ˲�õ�xֵ�������� �������������������� ���ƫ����ƫС����������
��5���ڵζ������У���������ƿ���ڱ�����ˮ��ϴһ�£�ԭ���ǣ��������������� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��ͬ���� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
|
|
�ң�H2C2O4��2H2O CO��+CO2��+3H2OΪ�жϼס��Һ�����ȷ���ڢ�֮���貹����ʵ��Ϊ �� ��
A������֤ʣ�������Ƿ��ȼ��ȼ��ʱ�������ɫ
B����ʣ�����廹ԭ�ȵ�CuO��ĩ���۲������ɫ�ı仯
C����ʣ������ͨ����ˮ�У�����ˮ�Ƿ���ɫ
D����ʣ����������ȼ�գ�������ˮ����ͭ����ȼ�ղ���
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com