
3.04 gͭþ�Ͻ���ȫ�ܽ���100 mL 14.0 mol/L��Ũ�����У��õ�NO2��N2O4�Ļ������2240 mL(��״��)����Ӧ�����Һ�м���2.0 mol/L NaOH��Һ������������ȫ������ʱ���õ�5.08 g����������˵������ȷ����
A���úϽ���ͭ��þ�����ʵ���֮����2��1
B������ԭ����������ʵ�����0.12 mol
C���õ�5.08 g����ʱ������NaOH��Һ�������700 mL
D��NO2��N2O4�Ļ�������У�NO2�����������80%

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ��2017���㽭ʡ����������ȫ������3��������ѡ����Ŀ����ѧ�Ծ��������棩 ���ͣ�������
ʵ������100 mL0.500mol��L-1��NaOH��Һ����CO2��һ��ʱ��ⶨCO2������������������������μ��뵽����CO2�����Һ�У�������CO2��������ʵ����������������ʾ��ͼ���£�
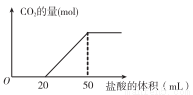
��(1)���յ�CO2�ڱ�״���µ������____mL��
(2)��������ʵ���Ũ����_______mol��L-1��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2016-2017ѧ��ӱ�ʡ�߶���ѧ��3���¿���ѧ�Ծ��������棩 ���ͣ�ѡ����
����ʹ���Ի����� ������ʧ�ķ�Ӧ��
������ʧ�ķ�Ӧ��
A. ������Ӧ B. ȡ����Ӧ C. �ӳɷ�Ӧ D. ��ȥ��Ӧ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2016-2017ѧ�갲��ʡ�����и�һ��ѧ�ڵ�һ�νμ�⻯ѧ�Ծ��������棩 ���ͣ������
I������ʯ����Ҫ�ɷ���K2SO4��Al2(SO4)3��2Al2O3��6H2O������������Fe2O3���ʣ�����������ʯ�Ʊ�����������
��1����֪����ʯ��ˮ��Ҫ����ѧ����ʽΪ2Al2(SO4)3+3S 2Al2O3+9SO2���÷�Ӧ���������뻹ԭ�������ʵ���֮����___________��������1 mol Al2O3����ת�Ƶĵ�����Ϊ________��
2Al2O3+9SO2���÷�Ӧ���������뻹ԭ�������ʵ���֮����___________��������1 mol Al2O3����ת�Ƶĵ�����Ϊ________��
��2��֤�������к���Fe2O3 �Ļ�ѧ������_____________________��
II��þ���Ͻ�(Mg17Al12 )��һ��DZ�ڵ�������ϣ�������������£���һ����ѧ�����ȵ�Mg��Al ������һ���¶���������á��úϽ���һ����������ȫ����ķ�Ӧ����ʽΪ��Mg17Al12 + 17H2=17MgH2 + 12Al ���õ��Ļ����Y(17MgH2 +12Al)��һ�������¿��ͷų�������
��3�������Ʊ�þ���Ͻ�(Mg17Al12)ʱͨ�������Ŀ����_____________________
��4����6.0 mol��L��1 HCl ��Һ�У������Y ����ȫ�ͷų�H2��1 mol Mg17Al12 ��ȫ�����õ��Ļ����Y ������������ȫ��Ӧ���ͷų�H2�����ʵ���Ϊ_______mol������֪��MgH2 + 2HCl = MgCl2 + 2H2����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2016-2017ѧ�갲��ʡ�����и�һ��ѧ�ڵ�һ�νμ�⻯ѧ�Ծ��������棩 ���ͣ�ѡ����
100mlij���Һ��HNO3�����ʵ���Ũ��Ϊ0.2mol/L�� H2SO4 �����ʵ���Ũ��Ϊ0.1mol/L�������м���1.92g Cu���ȣ���ȫ��Ӧ����Һ��Cu2+�����ʵ���Ũ��Ϊ( )
A. 0.3 mol/L B. 0.15 mol/L
C. 0.25 mol/L D. ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2016-2017ѧ�갲��ʡ�����и�һ��ѧ�ڵ�һ�νμ�⻯ѧ�Ծ��������棩 ���ͣ�ѡ����
����˵����ȷ���ǣ� ��
A. �����п�����������˵�����������������ġ�
B. ��ʳ����ϴ��ˮƿ���ڱڸ��ŵ�ˮ����˵�����������ǿ��̼������ԡ�
C. �մ��С�մ���ɫ��Ӧ����������ͬ�ģ�˵�������չ��������Ƿ�������ͬ�Ļ�ѧ�仯��
D. Һ���ܵ�й©����ʪ�����ɫʯ����ֽ���м��顣
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2016-2017ѧ�꽭��ʡ��һ��ѧ�����п��Ի�ѧ�Ծ��������棩 ���ͣ������
�±��г���10��Ԫ����Ԫ�����ڱ��е�λ�ã�

������Ӧ�Ļ�ѧ����ش��������⣺
��1��Ԫ�آ���Ԫ�����ڱ��д��ڵ�_____����,��______�塣
��2������10��Ԫ���У���������ǿ��Ԫ�صķ���Ϊ_________������������Ӧˮ�����У�������ǿ�����ʵķ���ʽΪ______�����к�Ar��������Ų���ͬ�����ӵİ뾶�ɴ�С��˳����Ϊ__________(�����ӷ��ű�ʾ)��
��3��Ԫ�آ۵�����⻯��ĵ���ʽΪ________��Ԫ�آں�Ԫ�آ�����⻯���У��ȶ��Խ�ǿ�����ʵĽṹʽΪ______���õ���ʽ��ʾ�ߺ͢��γɻ�����Ĺ���________��
��4�����ڿ�����ȼ�յIJ�����������ѧ����________��
A.���Ӽ� B.���Լ� C.�Ǽ��Լ�
��5��Ԫ�آܡ��ݡ��ߵ�����������Ӧˮ��������֮�䷢����Ӧ�����ӷ���ʽ�ֱ�Ϊ____��_______��________����ĵ�����������������Ӧˮ���ﷴӦ�Ļ�ѧ����ʽΪ________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2016-2017ѧ�꽭��ʡ��һ��ѧ�����п��Ի�ѧ�Ծ��������棩 ���ͣ�ѡ����
Ŀǰ���ʿռ�վ����CO2�����漰�ķ�ӦΪCO2+4H2 CH4+2H2O���÷�Ӧ������˵����ȷ����
CH4+2H2O���÷�Ӧ������˵����ȷ����
A. Ϊ���CO2��ת���ʣ����ʵ����H2��Ũ��
B. �����¶��ܼ����÷�Ӧ������
C. �ﵽƽ��ʱ��H2��CH4�����֮��Ϊ4 : l
D. �ﵽƽ��ʱ����1molH-H��ͬʱ�γ�1molO-H��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2016-2017ѧ�����ʡ�����и�һ��ѧ�ڵ�һ���¿���3�£���ѧ�Ծ��������棩 ���ͣ������
��Ҫ��������и��⣺
��1��ճ��[��Ҫ�ɷ�Al2Si2O5(OH)4] ���Ʊ��մɵ�ԭ�ϣ�������������ʽ��ʾճ�������_____________��
��2���������Ȼ���͵����������м�ǿ�ȷ�����Ӧ�����Ƶøߴ��ȵ����裬��Ӧ�Ļ�ѧ����ʽΪ_________________________��
��3���������������Բ������п�ʴ����һ�����з�����Ӧ�Ļ�ѧ����ʽΪ_______��
��4�����������ھ�ˮ����˵��ԭ��________________________��
��5����ͼ����A��B��C�о���ͬһ�ֳ�������Ԫ�أ���Ԫ����C������������ʽ���ڣ� B�ǰ�ɫ��״������
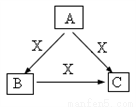
��ʵ�����г���A��ij����Һ��Ӧ�Ʊ�B,��д���÷�Ӧ�����ӷ���ʽ _________________��
��A��CҲ���Է�Ӧ����B,��д����Ӧ�����ӷ���ʽ___________________��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com