����ͼ��ʾ�Ƿ�������ʱ���õ��������ش��������⣺

��1�������A��C��E������
������ƿ����Һ©����������
������ƿ����Һ©����������
��2���������»����Ӧ����Ҫѡ��ʲô������������ĸ���ţ�
ʳ���ͺ;ƾ���
AE
AE
���ͺ�ˮ��
C
C
��ʵ�����ù����ռ�����200mL 0.5mol?L
-1��NaOH��Һ��
��1�������
5.0
5.0
g �ռӦ����
�ձ�
�ձ�
�г������ܽ⣮
��2����ɴ�����ʵ�飬������Ͳ���ձ������������Ҫ�ij����IJ���������
250mL����ƿ����ͷ�ι�
250mL����ƿ����ͷ�ι�
��3�����������ݲ���Ӧ��ȡ�ľ��巽��
������ƿ��ע������ˮ���̶���1��2cm�������ý�ͷ�ιܵμ�����ˮ����Һ�İ�Һ����̶�������ˮƽ����
������ƿ��ע������ˮ���̶���1��2cm�������ý�ͷ�ιܵμ�����ˮ����Һ�İ�Һ����̶�������ˮƽ����
��




 �Ͻ�ƽ��У����ϵ�д�
�Ͻ�ƽ��У����ϵ�д�
 ��1����ͼ��ʾ��U�ܵ���˱�ˮ�ͽ�������м���������������Ϊ1��4���Ļ�����壬�ٶ�������ˮ���ܽ�ȿ��Ժ��ԣ�������м���������Ļ�������װ�÷������й����ĵط����û�����建���ķ�Ӧһ��ʱ�䣮
��1����ͼ��ʾ��U�ܵ���˱�ˮ�ͽ�������м���������������Ϊ1��4���Ļ�����壬�ٶ�������ˮ���ܽ�ȿ��Ժ��ԣ�������м���������Ļ�������װ�÷������й����ĵط����û�����建���ķ�Ӧһ��ʱ�䣮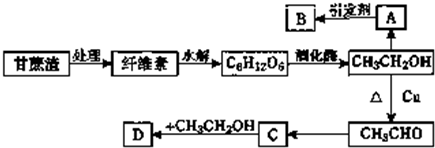
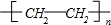
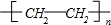
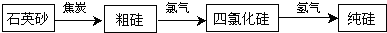
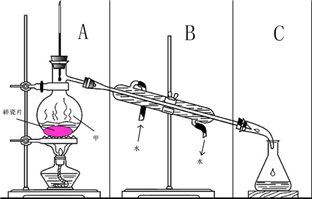 ��ͼ��ʾװ������ѧ��ѧʵ���г������ʵķ������ᴿ
��ͼ��ʾװ������ѧ��ѧʵ���г������ʵķ������ᴿ