



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
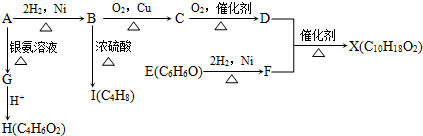
| O | ||
|
| O | ||
|
 CH3CH2CH2-
CH3CH2CH2-| O | ||
|
 CH3CH2CH2-
CH3CH2CH2-| O | ||
|
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ�갲��ʡ���������ѧ�߶���ѧ����������4��ѧ�Ծ����������� ���ͣ������
(12��)(2009��ɽ������)ZnMnO2�ɵ��Ӧ�ù㷺����������Һ��ZnCl2NH4Cl�����Һ��
(1)�õ�صĸ���������________����ع���ʱ����������________(�������������)��
(2)��ZnCl2NH4Cl�����Һ�к�������Cu2���������ij�缫�ĸ�ʴ������Ҫԭ����___ _____________________________________________________________________��
����ȥCu2�������ѡ�������Լ��е�________(�����)��
a��NaOH������������������ b��Zn
c��Fe d��NH3��H2O
(3)MnO2����������֮һ����ʯīΪ�缫������ữ��MnSO4��Һ�������ĵ缫��Ӧʽ��__________________��������·��ͨ��2 mol���ӣ�MnO2�����۲���Ϊ________g��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013�찲��ʡ�߶���ѧ����������4��ѧ�Ծ��������棩 ���ͣ������
(12��)(2009��ɽ������)ZnMnO2�ɵ��Ӧ�ù㷺����������Һ��ZnCl2NH4Cl�����Һ��
(1)�õ�صĸ���������________����ع���ʱ����������________(�������������)��
(2)��ZnCl2NH4Cl�����Һ�к�������Cu2���������ij�缫�ĸ�ʴ������Ҫԭ����___ _____________________________________________________________________��
����ȥCu2�������ѡ�������Լ��е�________(�����)��
a��NaOH������������������ b��Zn
c��Fe d��NH3��H2O
(3)MnO2����������֮һ����ʯīΪ�缫������ữ��MnSO4��Һ�������ĵ缫��Ӧʽ��__________________��������·��ͨ��2 mol���ӣ�MnO2�����۲���Ϊ________g��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com