
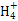 ���幹���� ,������ԭ�ӵ��ӻ���ʽΪ ��
���幹���� ,������ԭ�ӵ��ӻ���ʽΪ ��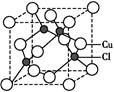
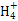 ����ԭ�ӵ�ԭ�Ӳ���sp3�ӻ�,�ռ乹��Ϊ����������;(3)��ˮ��Һ�а������ӺͰ�������֮������γ����,ˮ���Ӻ�ˮ����֮������γ����,ˮ���ӺͰ�������֮��Ҳ�����γ����(������ʽ),���ͨ���á�������ʾ;(4)������[Cu(NH3)4]2+ʹ��Һ������ɫ,�������к�����λ��;(5)��̯��,�þ����к���ͭԭ��8��
����ԭ�ӵ�ԭ�Ӳ���sp3�ӻ�,�ռ乹��Ϊ����������;(3)��ˮ��Һ�а������ӺͰ�������֮������γ����,ˮ���Ӻ�ˮ����֮������γ����,ˮ���ӺͰ�������֮��Ҳ�����γ����(������ʽ),���ͨ���á�������ʾ;(4)������[Cu(NH3)4]2+ʹ��Һ������ɫ,�������к�����λ��;(5)��̯��,�þ����к���ͭԭ��8�� +6��
+6�� =4��,��ԭ��4��,������1��1�Ĺ�ϵ,��˸��Ȼ���Ļ�ѧʽΪCuCl��
=4��,��ԭ��4��,������1��1�Ĺ�ϵ,��˸��Ȼ���Ļ�ѧʽΪCuCl��

 ��ְٷְټ�����Ԫ��ĩ���Ծ�ϵ�д�
��ְٷְټ�����Ԫ��ĩ���Ծ�ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ������

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
| | �� | �� | �� | �� | �� |
| ��һ������ ��kJ/mol�� | 1681 | 1251 | 1140 | 1008 | 900 |
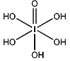 ����HIO4��ǰ��Ϊ��Ԫ�ᣬ����ΪһԪ�ᡣ��Ƚ϶�������ǿ����H5IO6_____HIO4�����������������������
����HIO4��ǰ��Ϊ��Ԫ�ᣬ����ΪһԪ�ᡣ��Ƚ϶�������ǿ����H5IO6_____HIO4�����������������������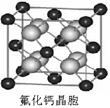
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
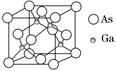
| A���黯�ؾ����ṹ��NaCl��ͬ |
| B���黯�ؾ�������ͬһ����ԭ����������ԭ�ӹ����������� |
| C���縺�ԣ�As>Ga |
| D���黯�ؾ����к�����λ�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
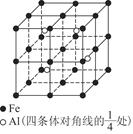
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
| ������/��kJ��mol��1�� | I1 | I2 | I3 | I4 |
| A | 932 | 1 821 | 15 390 | 21 771 |
| B | 738 | 1 451 | 7 733 | 10 540 |
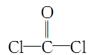 ��ÿ��COCl2�����ں���________���Ҽ���________���м���������ԭ�Ӳ�ȡ________�ӻ������ʽ��
��ÿ��COCl2�����ں���________���Ҽ���________���м���������ԭ�Ӳ�ȡ________�ӻ������ʽ���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������

| A��C3H8��̼ԭ�Ӷ����õ���sp3�ӻ� |
| B��O2��CO2��N2���ǷǼ��Է��� |
| C��ÿ��N2�У�����2���м� |
| D��CO��һ�ֵȵ�����ΪNO�������ĵ���ʽΪ[��N??O��]�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
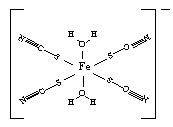

 NH4++NH2���������ӻ�����Ϊl��0��l0��30���ֽ�2��3g������Ͷ��1��0 LҺ���У�����ȫ��Ӧ����NaNH2��������Һ��������䣬������Һ��NH4+��Ũ��
NH4++NH2���������ӻ�����Ϊl��0��l0��30���ֽ�2��3g������Ͷ��1��0 LҺ���У�����ȫ��Ӧ����NaNH2��������Һ��������䣬������Һ��NH4+��Ũ��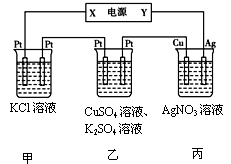
| A���������缫��Ӧʽ��Cu2++2e-��Cu |
| B�����һ��ʱ���װ�ñ���pH��С |
| C�������ͨ��������HCl���壬��ʹ��Һ�ָ������ǰ��״̬ |
| D�����һ��ʱ��������м���0.1molCu(OH)2��ʹ�������Һ��ԭ,���·��ͨ���ĵ���Ϊ0.2mol������������Һ����MgCl2,�����ܷ�Ӧ�����ӷ���ʽΪ�� |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com