
CO2��CH4��������Ҫ���������壬ͨ��CH4��CO2��Ӧ�������ֵ��ѧƷ��Ŀǰ���о�Ŀ�ꡣ
��250��ʱ�������Ͻ�Ϊ��������4 L������ͨ��6 mol CO2��6 mol
CH4���������·�Ӧ��CO2 (g)��CH4(g) 2CO(g)��2H2(g)��ƽ����ϵ�и��������������±���
2CO(g)��2H2(g)��ƽ����ϵ�и��������������±���
|
���� |
CH4 |
CO2 |
CO |
H2 |
|
������� |
0.1 |
0.1 |
0.4 |
0.4 |
�ٴ��¶��¸÷�Ӧ��ƽ�ⳣ��K= ��
����֪��CH4(g)��2O2(g)��CO2(g)��2H2O(g) ��H=-890.3 kJ��mol��1
CO(g)��H2O (g)��CO2(g)��H2 (g) ��H=2.8 kJ��mol��1
2CO(g)��O2(g)��2CO2(g) ��H=-566.0 kJ��mol��1
��ӦCO2(g)��CH4(g) 2CO(g)��2H2(g)
�ġ�H= ��
2CO(g)��2H2(g)
�ġ�H= ��
���Զ������ѱ��渲��Cu2Al2O4Ϊ���������Խ�CO2��CH4ֱ��ת�������ᡣ
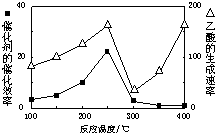
���ڲ�ͬ�¶��´����Ĵ�Ч���������������������ͼ��ʾ��250��300��ʱ���¶����߶�������������ʽ��͵�ԭ���� ��
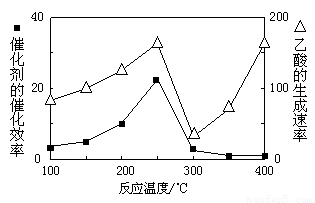
��Ϊ����߸÷�Ӧ��CH4��ת���ʣ����Բ�ȡ�Ĵ�ʩ�� ��
�۽�Cu2Al2O4�ܽ���ϡ�����е����ӷ���ʽΪ ��
����CO2Ϊԭ�Ͽ��Ժϳɶ������ʡ�
�پ�̼������һ����������ͺϳɲ��ϣ����������۶��ɡ�д����̼�����Ľṹ��ʽ�� ��
������������ˮ��Һ������ʽ��е�⣬CO2��ͭ�缫�Ͽ�ת��Ϊ���飬�õ缫��Ӧ����ʽΪ ��
��64 ��+247.3 kJ��mol��1
�Ƣ��¶ȳ���250��ʱ�������Ĵ�Ч�ʽ���
������Ӧѹǿ������CO2��Ũ��
��3Cu2Al2O4+32H++2NO3��=6Cu2++ 6Al3++2NO��+16 H2O
�Ǣ�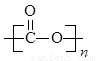 ��CO2+8e��+6H2O=CH4+8OH��
��CO2+8e��+6H2O=CH4+8OH��
��ÿ��2�֣���14�֣�
��������
�����������1���� CO2 (g)��CH4(g) 2CO(g)��2H2(g)
2CO(g)��2H2(g)
��ʼŨ�ȣ�mol/L�� 1.5 1.5 0 0
ת��Ũ�ȣ�mol/L�� x x 2x 2x
ƽ��Ũ�ȣ�mol/L�� 1.5��x 1.5��x 2x 2x
��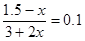
���x��1
���Ը÷�Ӧ��ƽ�ⳣ��K��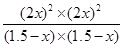 ��64��
��64��
�ڸ��ݸ�˹���ɿ�֪���٣��ڡ�2���ۡ�2�����õ���ӦCO2(g)��CH4(g) 2CO(g)��2H2(g)�����Ը÷�Ӧ�ġ�H=��890.3 kJ/mol��2.8 kJ/mol����566 kJ/mol��2����247.3 kJ/mol��
2CO(g)��2H2(g)�����Ը÷�Ӧ�ġ�H=��890.3 kJ/mol��2.8 kJ/mol����566 kJ/mol��2����247.3 kJ/mol��
��2�������ڴ����Ĵ�Ч�����¶�Ӱ�����˵��¶ȳ���250��ʱ�������Ĵ�Ч�ʽ��ͣ��Ӷ�����������������ʽ��͡�
�����ڸ÷�Ӧ�������С�Ŀ��淴Ӧ������Ϊ����߸÷�Ӧ��CH4��ת���ʣ����Բ�ȡ�Ĵ�ʩ������Ӧѹǿ������CO2��Ũ�ȡ�
�������Һǿ�����ԣ����Խ�Cu2Al2O4�ܽ���ϡ�����е����ӷ���ʽΪ3Cu2Al2O4��32H+��2NO3����6Cu2++ 6Al3+��2NO����16 H2O��
��3���پ�̼������һ����������ͺϳɲ��ϣ����������۶��ɣ��䵥����̼�ᣬ���Ծ�̼�����Ľṹ��ʽ��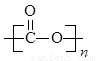 ��
��
��CO2��ͭ�缫�Ͽ�ת��Ϊ���飬˵��ͭ�缫��������������ԭ��Ӧ���������������ǵ�������ף���˵缫��Ӧʽ��CO2+8e��+6H2O=CH4+8OH����
���㣺���黯ѧƽ�ⳣ������Ӧ�ȵļ��㡢��������Է�Ӧ���ʵ�Ӱ���Լ����۷�Ӧ�͵缫��Ӧʽ����д��
�����������Ǹ߿��еij�����������ͣ������е��Ѷ�����Ŀ��飬�����ۺ���ǿ�����ض�ѧ�������������ͽ��ⷽ����ָ����ѵ����ּ�ڿ���ѧ��������û���֪ʶ���ʵ�����������������������ѧ���������������ͷ�ɢ˼ά����������ѧ����ѧ��������


 ��У����ϵ�д�
��У����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
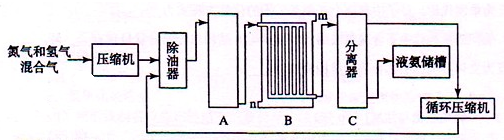
| ||
| ||
| ||
| �� |
| ||
| �� |
| ���¡���ѹ |
| ���� |
| ���¡���ѹ |
| ���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| ||
| ||
| ||
| ||
| FeBr3 |
| FeBr3 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
 ��2013?������ģ��CO2��CH4��������Ҫ���������壬ͨ��CH4��CO2��Ӧ�������ֵ��ѧƷ��Ŀǰ���о�Ŀ�꣮
��2013?������ģ��CO2��CH4��������Ҫ���������壬ͨ��CH4��CO2��Ӧ�������ֵ��ѧƷ��Ŀǰ���о�Ŀ�꣮| ���� | CH4 | CO2 | CO | H2 |
| ������� | 0.1 | 0.1 | 0.4 | 0.4 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012-2013ѧ�꽭��ʡ��ͨ�и���������ģ�⻯ѧ�Ծ��������棩 ���ͣ������
CO2��CH4��������Ҫ���������壬ͨ��CH4��CO2��Ӧ�������ֵ��ѧƷ��Ŀǰ���о�Ŀ�ꡣ
��1��250��ʱ�������Ͻ�Ϊ��������4 L������ͨ��6 mol CO2��6 mol CH4���������·�Ӧ��CO2(g)��CH4(g) 2CO(g)��2H2(g)��ƽ����ϵ�и��������������±���
2CO(g)��2H2(g)��ƽ����ϵ�и��������������±���
|
���� |
CH4 |
CO2 |
CO |
H2 |
|
������� |
0.1 |
0.1 |
0.4 |
0.4 |
�ٴ��¶��¸÷�Ӧ��ƽ�ⳣ��K= ��
����֪��CH4(g)��2O2(g)��CO2(g)��2H2O(g) ��H=-890.3 kJ��mol��1
CO(g)��H2O (g)��CO2(g)��H2 (g) ��H=2.8 kJ��mol��1
2CO(g)��O2(g)��2CO2(g) ��H=-566.0 kJ��mol��1
��ӦCO2(g)��CH4(g) 2CO(g)��2H2(g)
�ġ�H= ��
2CO(g)��2H2(g)
�ġ�H= ��
��2���Զ������ѱ��渲��Cu2Al2O4Ϊ���������Խ�CO2��CH4ֱ��ת�������ᡣ
���ڲ�ͬ�¶��´����Ĵ�Ч�������������������ͼ��ʾ��250��300��ʱ���¶����߶�������������ʽ��͵�ԭ���� ��
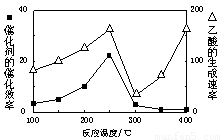
��Ϊ����߸÷�Ӧ��CH4��ת���ʣ����Բ�ȡ�Ĵ�ʩ�� ��
�۽�Cu2Al2O4�ܽ���ϡ�����е����ӷ���ʽΪ ��
��3����CO2Ϊԭ�Ͽ��Ժϳɶ������ʡ�
�پ�̼������һ����������ͺϳɲ��ϣ������ɼӾ۶��ɡ�д����̼�����Ľṹ��ʽ�� ��
������������ˮ��Һ������ʽ��е�⣬CO2��ͭ�缫�Ͽ�ת��Ϊ���飬�õ缫��Ӧ����ʽΪ ��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com