
(1)�������С���Ѿ������˻�ѧ��Ӧ�ķ�Ӧ���д�������Ļ�ѧ��Ӧ����ʽ����ƽ����ÿС��2�֣���6�֣�
������������͵�������Ӧ
����ˮ�⣺
CH3CH(OH)CH3�Ĵ�������
(2)��4�֣�.��-��ѭ���ֽ�ˮ������Ҫ�漰���з�Ӧ��
��.SO2+2H2O+I2===H2SO4+2HI
��.2HI H2+I2 ��.2H2SO4===2SO2+O2+2H2O
H2+I2 ��.2H2SO4===2SO2+O2+2H2O
��1��һ���¶��£���1L�ܱ������м���1mol HI��g����������Ӧ�����ɵ�I2Ϊ���壬H2���ʵ�����ʱ��ı仯��ͼ��ʾ�� 0-2 min�ڵ�ƽ����Ӧ���ʦԣ�HI��=
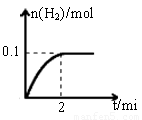
��2��ʵ������Zn��������ȡH2��Ϊ�˼ӿ췴Ӧ���ʣ����д�ʩ�����е��� ������ţ�
a������Ũ���� b����������CuSO4 ���� c���ô�п���洿п
d������ e.��п��Ū��п�� f.��98.3%Ũ����
(1) ��ÿС��3�֣���6�֣�
3CH3COOH��CH2OHCHOHCH2OH
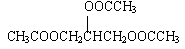 ��3H2O
��3H2O
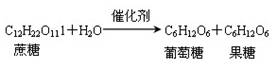
2CH3CHOHCH3��O2 2CH3COCH3��2H2O
2CH3COCH3��2H2O
(2)�����֣���1��0.1mol/(L��min)---2��, ��2��a��f--2��
����������1�������к���3���ǻ������Ժ����ᷢ��������Ӧ������3�������ᡣ�����Ƕ��ǣ�ˮ�����������Ǻ��ǡ�2�������к��ǻ�������̼ԭ����ֻ��1����ԭ�ӣ��������IJ����DZ�ͪ��
��2������ͼ���֪��ƽ��ʱ���������ʵ�����0.1mol���������ĵ⻯����0.2mol������䷴Ӧ������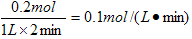 ��
��
��3��������������Է�Ӧ���ʵ�Ӱ�졣Ũ�����Ũ���ᶼ���������ᣬ��п��Ӧ�����ﲻ��������b��c�п��Թ���ԭ��أ���Ӧ���ʿ��Լӿ졣�¶����ߣ���Ӧ���ʼӿ졣e������Ӧ��ĽӴ��������Ӧ���ʼӿ졣���Դ�ѡaf��


 ѧ�����νӽ̲��Ͼ���ѧ������ϵ�д�
ѧ�����νӽ̲��Ͼ���ѧ������ϵ�д� Сѧ������ҵϵ�д�
Сѧ������ҵϵ�д� ��ʿһ��ȫͨϵ�д�
��ʿһ��ȫͨϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ��ɽ��ʡ�ٷ�һ�и�һ6���¿���ѧ�Ծ����������� ���ͣ������
(1)�������С���Ѿ������˻�ѧ��Ӧ�ķ�Ӧ���д�������Ļ�ѧ��Ӧ����ʽ����ƽ����ÿС��2�֣���6�֣�
������������͵�������Ӧ
����ˮ�⣺
CH3CH(OH)CH3�Ĵ�������
(2)��4�֣�.��-��ѭ���ֽ�ˮ������Ҫ�漰���з�Ӧ��
��.SO2+2H2O+I2===H2SO4+2HI ��.2HI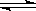 H2+I2 ��.2H2SO4===2SO2+O2+2H2O
H2+I2 ��.2H2SO4===2SO2+O2+2H2O
��1��һ���¶��£���1L�ܱ������м���1mol HI��g����������Ӧ�����ɵ�I2Ϊ���壬H2���ʵ�����ʱ��ı仯��ͼ��ʾ��0-2 min�ڵ�ƽ����Ӧ���ʦԣ�HI��= 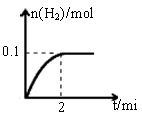
��2��ʵ������Zn��������ȡH2��Ϊ�˼ӿ췴Ӧ���ʣ����д�ʩ�����е��� ������ţ�
a������Ũ���� b����������CuSO4���� c���ô�п���洿п
d������ e.��п��Ū��п�� f.��98.3%Ũ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ�����ʡ��ˮһ�и�һ5���¿���ѧ�Ծ����������� ���ͣ������
�������С���Ѿ������˻�ѧ��Ӧ�ķ�Ӧ���д�������Ļ�ѧ��Ӧ����ʽ����ƽ��
��ÿС��3�֣���9�֣�
��ϩ�Ʊ�����ϩ��
��ϩ��ˮ��Ӧ��
�������ᷴӦ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2014�����ʡ��һ5���¿���ѧ�Ծ��������棩 ���ͣ������
�������С���Ѿ������˻�ѧ��Ӧ�ķ�Ӧ���д�������Ļ�ѧ��Ӧ����ʽ����ƽ��
��ÿС��3�֣���9�֣�
��ϩ�Ʊ�����ϩ��
��ϩ��ˮ��Ӧ��
�������ᷴӦ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�������С���Ѿ������˻�ѧ��Ӧ�ķ�Ӧ���д�������Ļ�ѧ��Ӧ����ʽ����ƽ��
��ÿС��3�֣���9�֣�
��ϩ�Ʊ�����ϩ��
��ϩ��ˮ��Ӧ��
�������ᷴӦ��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com