
��Ԫ�صĺ����������ᡢ�����ᡢ������Ⱥܶ��֣�����������(H3PO3)�Ǿ���ǿ��ԭ�ԵĶ�Ԫ���ᣬ���Ա�����������Ϊ���ᡣ
��1��д��������������NaOH��Һ��Ӧ�����ӷ���ʽ____________________________��
��2���������������ӷ�Ӧʱ�������뻹ԭ�������ʵ���֮��Ϊ______________��
��3��ij�¶��£�0.10mol•L-1��H3PO3��Һ��pHΪ1.6����c(H+)=2.5��10-2mol•L-1�����¶���H3PO3�ĵ���ƽ�ⳣ��K=___________________��(H3PO3�ڶ���������Բ��ƣ��������������Ч����)
��4����H3PO3��Һ�еμ�NaOH��Һ�����ԣ�������Һ�У�c(Na+)_____c(H2PO3-)+2c(HPO32-)(���������������=������ͬ)����NaH2PO3��Һ�У�c(H+)+c(H3PO3)_____c(HPO32-)+c(OH-)
��5�����Na2HPO3��ҺҲ�ɵõ������ᣬװ��ʾ��ͼ���£�
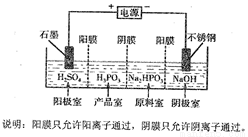
�ٲ�Ʒ���з�Ӧ�����ӷ���ʽΪ_______________________��
�ڵõ�1mol�������ͬʱ���������Ƶ�NaOH����Ϊ________g��
��6����֪��εķֽ�Ƚϸ��ӣ����ֽ�ʱ���漰�����ϼ۱仯����ηֽ�Ƚϼ���ʵ�ʾ������ӵ�ת��(��NH4A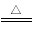 NH3+HA)�������Ӧ�ĸ�����ηֽ�ʱ���漰���ϼ۱仯���Դ�������ӽ�����������Ľ�ȣ��ж�������εķֽ��¶ȣ�
NH3+HA)�������Ӧ�ĸ�����ηֽ�ʱ���漰���ϼ۱仯���Դ�������ӽ�����������Ľ�ȣ��ж�������εķֽ��¶ȣ�
NH4H2PO4____________(NH4)2HPO4(���������������=��)
 ��ĩ1�����ʽ���������ϵ�д�
��ĩ1�����ʽ���������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ��2015-2016ѧ���Ĵ�ʡ��һ�µڶ��ζο���ѧ���������棩 ���ͣ�ѡ����
������һ����Դ��������Ҫ�ɷ���CH4,0.5 mol CH4��ȫȼ������CO2��H2Oʱ���ų�
445 kJ�����������ʾCH4ȼ���ȵ��Ȼ�ѧ����ʽ��ȷ����
A.1/2CH4��g����O2��g���� 1/2CO2��g����H2O ��l�� ��H =��445 kJ/mol
B.CH4��2O2��CO2��H2O ��H=��890 kJ/mol
C.CH4��g����2O2��g����CO2��g����2 H2O ��l�� ��H =��890 kJ/mol
D.CH4��g����2O2��g����CO2��g����2H2O ��l�� ��H = +890 kJ/mol
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2016�����ʡ����5��ģ�������ۻ�ѧ�Ծ��������棩 ���ͣ������
ij���᳦��ҩ����Ч�ɷֵĺϳ�·������(���ַ�Ӧ��ȥ�Լ�������)��
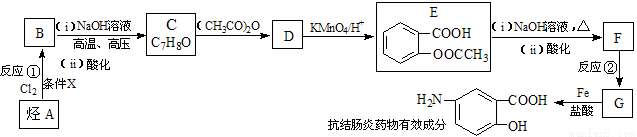
��֪��

��ش��������⣺
��1�����᳦��ҩ����Ч�ɷֵķ���ʽ�� ����Ӧ�ٵķ�Ӧ������ ����Ӧ�ڵķ�Ӧ������ ��
��2�������жԸÿ��᳦��ҩ����Ч�ɷֿ��ܾ��е������Ʋ���ȷ���� ��
A��ˮ���Աȱ��Ӻ�
B���ܷ�����ȥ��ӦҲ�ܷ����ۺϷ�Ӧ
C��1mol������������3mol�巢����Ӧ
D���������������
��E������NaOH��Һ��Ӧ�Ļ�ѧ����ʽ�� ��
��3���������������Ŀ��᳦��ҩ����Ч�ɷֵ�ͬ���칹����______��.
A����FeCl3��Һ����ɫ��Ӧ��B�������м��뱽��ֱ��������C�������Ϲ�������ȡ����
��4����֪����( )�ױ��������������������ʱ������һ��ȡ��������ȡ����������ڶ�λ�����������������Ȼ�ʱ��ȡ���ڼ�λ.�ݴ˰��Ⱥ�˳��д����AΪԭ�Ϻϳ��ڰ���������(
)�ױ��������������������ʱ������һ��ȡ��������ȡ����������ڶ�λ�����������������Ȼ�ʱ��ȡ���ڼ�λ.�ݴ˰��Ⱥ�˳��д����AΪԭ�Ϻϳ��ڰ���������(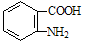 )�ϳ�·���������м����Ľṹ��ʽ(���ַ�Ӧ��������ȥ)
)�ϳ�·���������м����Ľṹ��ʽ(���ַ�Ӧ��������ȥ)
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2016��ӱ�ʡ������ѧ�ڲ������ۻ�ѧ�Ծ��������棩 ���ͣ�ʵ����
(NH4)2Fe(SO4)2��6H2O(Ī���Σ�dz��ɫ��ʽ��392)�ڶ��������г������궨������ء��ظ���ص���Һ�ı����ʣ���������ѧ�Լ���ҽҩ�Լ�����ұ�𡢵�Ƶȡ�
�ش��������⣺
��1��Ī�����ڿ����б����������ȶ���������¶���ڿ�����Ҳ����ʣ�����Ī�����Ƿ���ʵ��Լ���_______________��
��2��ȷ��ȡmg������Ī���Σ�����ƿ�м���20mLˮ����ܽ⣬��ij����K2Cr2O7��Һ�ζ����յ㡣�ظ�����3�Σ�����й��������£�
ʵ����� | ��ʼ����/mL | �յ����/mL |
I | 2.50 | 22.58 |
�� | 1.00 | 23.12 |
�� | 0.00 | 19.92 |
��K2Cr2O7��ҺӦ�÷���______________ʽ�ζ����С�
��д���ζ������з�Ӧ�����ӷ���ʽ��______________��
������K2Cr2O7��Һ�����ʵ���Ũ��Ϊ______________mol/L(�ú�M�Ĵ���ʽ��ʾ)��
��3��ij������ͨ��ʵ�����Ī���ξ������ʱ�ķֽ���
�ټ�ͬѧ������룺�ֽ���������N2��Fe2O3��SO3��H2O�������ʡ����Ƿ�ͬ�Ⲣ˵�����ɣ�______________��
����ͬѧ�������ͼװ�ã�����Aװ���еĹ����Ϊ����ɫ�����������к���______________��Cװ���к�ɫ��ȥ��˵����������к���______________��
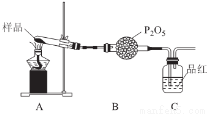
Cװ�ú�Ӧ����β������װ��D��D��ʢ�е��Լ�������______________(дһ�ּ���)��
�۱�ͬѧ����������װ��֤���ֽ�����к��а���.ֻ�����B��C�е��Լ����ɣ����������Լ�ΪB______________��C______________��
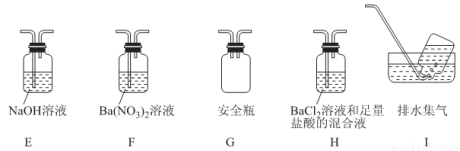
�ܶ�ͬѧ��ΪĪ���ηֽ���ܻ�����N2��SO3���������װ����ѡ���Ҫ��װ�ü���֤��������ȷ������˳�������������A��______________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2016��ӱ�ʡ������ѧ�ڲ������ۻ�ѧ�Ծ��������棩 ���ͣ�ѡ����
��ѧ�������ᷢչϢϢ���.����˵����ȷ���ǣ� ��
A������ɰ(HgS)��֮��ˮ���������ֻ����˵�ɰ�����ù��̷�����������ԭ��Ӧ
B������ӻ�������֬��������ˮ��Ϊ��������͵�С���Ӳ��ܱ�����
C������ֲ���͵�������Ӧ���Ի������֬��
D��������轺������Ҫ�ɷֶ��Ƕ�������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2016��ɽ��ʡ�����߿���в������ۻ�ѧ�Ծ��������棩 ���ͣ�ѡ����
�ܻ���(DCHP)���������ϼӹ�������һ���Ʊ��������£�
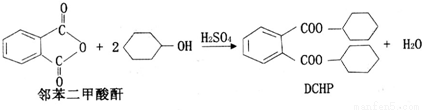
����˵����ȷ����( )
A��DCHP�ķ���ʽΪC20H28O4
B�������Ʊ�DCHP�ķ�Ӧ����ȡ����Ӧ
C��DCHP�����ϵ�һ�ȴ�����4��
D��1molDCHP�����뺬4molNaOH����Һ��Ӧ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2016������ʡ����ȫ��ģ�����ۻ�ѧ�Ծ��������棩 ���ͣ�ʵ����
���Ȼ���(SO2Cl2)��һ����Ҫ���л��ϳ��Լ���ʵ���ҿ�����SO2��Cl2��Ӧ��ȡ������SO2Cl2��װ����ͼ(��Щ֧��װ��ʡ����)��ʾ��
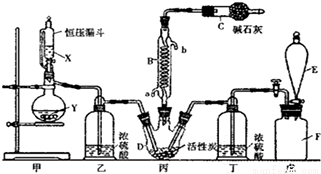
��֪��SO2Cl2���۵�Ϊ-54.1�棬�е�Ϊ69.1�棬��ˮ�ܷ������ҵ�ˮ�ⷴӦ������֮һΪ�Ȼ������塣
��1��E�е��Լ��DZ���ʳ��ˮ����������װ�ã���Fƿ�������ʵ�����Ʒ���_______(�÷���ʽ��ʾ)��
��2��B����Ӧ����ȴˮӦ��___________(�a����b��)�ӿ�ͨ�룻
��3��װ���ҺͶ���������___________��
��4����ѹ©����������Ľṹ����ҪĿ����___________��
��5��д�����Ȼ���(SO2Cl2)ˮ�ⷴӦ�ķ���ʽ___________��
��6��SO2����ˮ���������ᣬ�����������ǿ�ڴ����ᣬѡ�������װ�ú�ҩƷ̽����������������������ǿ��

װ������˳��ΪA��_____��_____��_____��D��F������װ��C��������_____��ͨ��__________________________����֤�������������ǿ�ڴ����ᡣ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2016�콭��ʡ������ǰ�������ۻ�ѧ�Ծ��������棩 ���ͣ�ѡ����
��������һ�����ȼ�ϣ���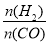 =2ͨ��1L�ķ�Ӧ���У�һ�������·�����Ӧ:2CO(g)+4H2(g)
=2ͨ��1L�ķ�Ӧ���У�һ�������·�����Ӧ:2CO(g)+4H2(g)  CH3OCH3(g) +H2O(g) ��H����CO��ƽ��ת�������¶ȡ�ѹǿ�仯��ϵ��ͼ��ʾ������˵����ȷ����
CH3OCH3(g) +H2O(g) ��H����CO��ƽ��ת�������¶ȡ�ѹǿ�仯��ϵ��ͼ��ʾ������˵����ȷ����
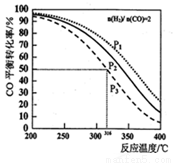
A��������Ӧ��H>0
B��ͼ��P1<P2<P3
C������P3��316��ʱ�����������n(H2)=n(CH3OCH3)����ʱv(��)<v(��)
D������P3��316��ʱ����ʼʱ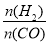 =3����ﵽƽ��ʱ��COת����С��50%
=3����ﵽƽ��ʱ��COת����С��50%
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2015-2016ѧ��ɽ��ʡ�߶�6���¿���ѧ�Ծ��������棩 ���ͣ�ѡ����
����ͼ��ʾװ�ü�����ϩʱ����Ҫ���ӵ� �ǣ� ��
�ǣ� ��
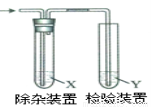
��ϩ���Ʊ� | �Լ�X | �Լ�Y | |
A | CH3CH2Br��NaOH�Ҵ���Һ���� | H2O | KMnO4������Һ |
B | CH3CH2Br��NaOH�Ҵ���Һ���� | H2O | Br2��CCl4��Һ |
C | CH3CH2OH�� | NaOH��Һ | KMnO4������Һ |
D | CH3CH2OH��ŨH2SO4������170�� | NaOH��Һ | Br2��CCl4��Һ |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com