
��֪��
A��B��C��D��EΪ���ڱ�1~36���е�Ԫ�أ����ǵ�ԭ������������A�Ļ�̬ԭ����3����ͬ���ܼ������ܼ��е�������ȣ�C�Ļ�̬ԭ��2p�ܼ��ϵ�δ�ɶԵ�������Aԭ����ͬ��C2������D2+���Ӿ�����ͬ�ġ��ȶ��ĵ��Ӳ�ṹ��E�Ļ�̬ԭ�ӵ���Χ�����Ų�ʽΪ3d84s2����ش��������⣺
(1)A��B��C��D����Ԫ���У��縺��������
________(��Ԫ�ط���)��(2)
B���⻯��ķе�Զ����A���⻯�����Ҫԭ����________��(3)
��A��B��C�γɵ�����CAB����AC2��Ϊ�ȵ����壬��CAB����Aԭ�ӵ��ӻ���ʽΪ________��(4)E2
+��������AC�����γ�[E(AC)4]2+����ԭ����AC�����к���________��(5)
������֣�ֻ��A��D��E����Ԫ�ص�һ�־���(������ͼ��ʾ)���г����ԣ�Aԭ�ӵ���λ��Ϊ________���þ���Ļ�ѧʽΪ________��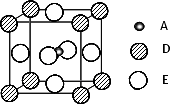

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ��2011?�����ģ����ѧ--ѡ�����ʽṹ������
��2011?�����ģ����ѧ--ѡ�����ʽṹ�������鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
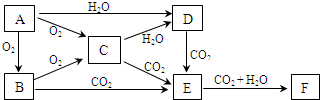
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
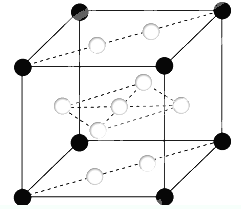 ��֪��A��B��C��D��E��F��XΪ���ڱ���ǰ�����ڵ�����Ԫ�أ����ǵ�ԭ��������������A�����ڱ���ԭ�Ӱ뾶��С��Ԫ�أ�B�Ļ�̬ԭ����3����ͬ���ܼ������ܼ��е�������ȣ�D�Ļ�̬ԭ��2p�ܼ��ϵ�δ�ɶԵ�������Bԭ�ӵ���ͬ��D2-������E2+���Ӿ�����ͬ���ȶ����Ӳ�ṹ��F�С����������֮�ƣ�F4+���Ӻ��ԭ�ӵĺ�������Ų���ͬ��X�Ļ�̬ԭ�ӵļ۵����Ų�ʽΪ3d84s2��
��֪��A��B��C��D��E��F��XΪ���ڱ���ǰ�����ڵ�����Ԫ�أ����ǵ�ԭ��������������A�����ڱ���ԭ�Ӱ뾶��С��Ԫ�أ�B�Ļ�̬ԭ����3����ͬ���ܼ������ܼ��е�������ȣ�D�Ļ�̬ԭ��2p�ܼ��ϵ�δ�ɶԵ�������Bԭ�ӵ���ͬ��D2-������E2+���Ӿ�����ͬ���ȶ����Ӳ�ṹ��F�С����������֮�ƣ�F4+���Ӻ��ԭ�ӵĺ�������Ų���ͬ��X�Ļ�̬ԭ�ӵļ۵����Ų�ʽΪ3d84s2��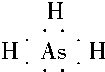
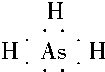
| 3 |
| ||
| 3 |
| ||
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ��֪����A��B��C��D�����µķ�Ӧ��ϵ������A ��B��ȼ��ʱ������ʲ�ɫ�� C��B��ȼ��ʱ�����ػ�ɫ���̣�E��ˮ��Һ������ɫ��G��һ�ֺ�ɫ���壮
��֪����A��B��C��D�����µķ�Ӧ��ϵ������A ��B��ȼ��ʱ������ʲ�ɫ�� C��B��ȼ��ʱ�����ػ�ɫ���̣�E��ˮ��Һ������ɫ��G��һ�ֺ�ɫ���壮
| ||
| ||
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com