
��ͼ��ʾװ���У��ס��ҡ��������ձ����ηֱ�ʢ��100 g 5.00%��NaOH��Һ��������CuSO4��Һ��100 g 10.00%��K2SO4��Һ���缫��Ϊʯī�缫��
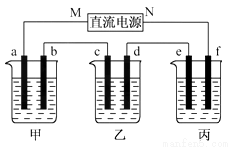
(1)��ͨ��Դ������һ��ʱ���ñ���K2SO4Ũ��Ϊ10.47%������c�缫�������ӡ��ݴ˻ش����⣺
�ٵ�Դ��N��Ϊ____________����
�ڵ缫b�Ϸ����ĵ缫��ӦΪ____________________��
����ʽ����缫b�����ɵ������ڱ�״���µ������________________��
�ܵ缫c�������仯��__________g��
�ݵ��ǰ�����Һ���ᡢ���Դ�С�Ƿ����仯��������ԭ��
����Һ__________________������Һ___________________������Һ______________��
(2)�����������ͭȫ����������ʱ����ܷ�������У�Ϊʲô�� _____________________��

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ��2017�츣��ʡ�����а�У������ѧ����ĩ������ѧ�Ծ��������棩 ���ͣ�ѡ����
�����й�˵����ȷ���ǣ� ��
A. ʵ�����У����ý����Ƽ����Ҵ����Ƿ���ˮ
B. ��������Ȼ������Ҫ�ɷ֣��ܷ���ȡ����Ӧ�������ܷ���������Ӧ
C. ֻ��ˮ�����𱽡�������Ě⻯̼
D. ֲ���Ͳ���������ȡĮˮ�е���
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2016-2017ѧ�����ʡ�˲��и߶���ѧ����ĩ���Ի�ѧ�Ծ��������棩 ���ͣ�ѡ����
���й����Ȼ�ѧ��Ӧ����������ȷ����
A. HCl��NaOH��Ӧ���к��Ȧ�H����57.3kJ/mol����H2SO4��Ca(OH)2��Ӧ���к��Ȧ�H��2��(��57.3)kJ/mol
B. CO(g)��ȼ������283.0kJ/mol����2CO2(g)��2CO(g)��O2(g)��Ӧ�Ħ�H��+566.0kJ/mol
C. ��Ҫ���Ȳ��ܷ����ķ�Ӧһ�������ȷ�Ӧ
D. 1mol����ȼ��������̬ˮ�Ͷ�����̼���ų��������Ǽ���ȼ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2017��ӱ�ʡ������ѧ����ĩ�������ۻ�ѧ�Ծ��������棩 ���ͣ������
ͭ���仯�������������������Ź㷺����;��
(1)��̬ͭԭ�ӵĺ�������Ų�ʽΪ_____________���侧��Ķѻ���ʽΪ__________________��
����ͭԭ�ӵ���λ��Ϊ_____________��
(2)������ͭ��Һ�еΰ�ˮ�������γ���ɫ�����������μӰ�ˮ�������ܽ⣬�õ�����ɫ������Һ����������Һ�м����Ҵ�������������ɫ����[Cu(NH3)4SO4��H2O]��
�ٰ�ˮ�и�Ԫ��ԭ�ӵĵ縺���ɴ�С��˳��Ϊ_______________(��Ԫ�ط��ű�ʾ)��
��NH3��Nԭ�ӵ��ӻ��������Ϊ_____________�����以Ϊ�ȵ������������Ϊ__________��
������ɫ�����м����μӰ�ˮ�������ܽ�����Ϊ�������İ���ͭ�����ӣ��İ���ͭ�����ӵĽṹʽ
Ϊ____________�������Ҵ������������ԭ��Ϊ________________��
(3) CuCl2��CuCl��ͭ�����ֳ������Ȼ��
����ͼ��ʾ����________________ (�CuCl2����CuCl��)�ľ�����

��ԭ�������������ʾ�����ڲ���ԭ�ӵ����λ�ã���ͼ�и�ԭ���������AΪ(0,0,0)��BΪ(0,1,1)��CΪ��1��1��0)����Dԭ�ӵ��������Ϊ____________��
����ͼ������C��D��ԭ�Ӻ˼��Ϊ298 pm,����٤������ΪNA����þ����ܶ�Ϊ______g��cm-3(�г�����ʽ���ɣ���
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2017��ӱ�ʡ������ѧ����ĩ�������ۻ�ѧ�Ծ��������棩 ���ͣ�ѡ����
�л���M��N��Q֮���ת����ϵΪ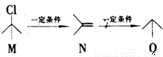 ������˵����ȷ����
������˵����ȷ����
A. M��ͬ���칹����3��(�����������칹��
B. N����������ԭ�ӹ�ƽ��
C. Q�����������
D. M��N��Q������Br2��Ӧ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2016-2017ѧ�����ʡ��ˮ�и߶���ѧ�ڿ�ѧ���ԣ�������ҵ��⣩��ѧ�Ծ��������棩 ���ͣ�ѡ����
һ���¶��£�������Һ������Ũ�ȹ�ϵʽ��ȷ����
A. pH=5��H2S��Һ�У�c(H+)= c(HS��)=1��10��5 mol��L��1
B. pH=a�İ�ˮ��Һ��ϡ��10������pH=b����a=b+1
C. pH=2��H2C2O4��Һ��pH=12��NaOH��Һ���������ϣ�c(Na��)+ c(H��)= c(OH��)+c( HC2O4��)
D. pH��ͬ�Ģ�CH3COO Na��NaHCO3��NaClO������Һ��c(Na��)���٣��ڣ���
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2016-2017ѧ�����ʡ��ˮ�и߶���ѧ�ڿ�ѧ���ԣ�������ҵ��⣩��ѧ�Ծ��������棩 ���ͣ�ѡ����
����ʵ��������������ǣ�������
A. ����ʪ��pH��ֽ��ϡ����Һ��pH���ⶨֵƫС
B. ������ƿ������Һ������ʱ���ӿ̶��ߣ�������ҺŨ��ƫС
C. �ζ�ǰ�ζ����������ݣ��յ����ʱ�����ݣ��������ƫС
D. �ⶨ�кͷ�Ӧ�ķ�Ӧ��ʱ��������������У������¶�ֵƫС
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2016-2017ѧ�꽭��ʡ�������и߶������ʰࣩ��ѧ����ĩ���Ի�ѧ�Ծ��������棩 ���ͣ�ѡ����
�����ӵ�������NA��ʾ��������������ȷ���ǣ� ��
�ٱ�״���£�22.4L�Ҵ��к��е���ԭ����ĿΪNA
��6.4g��34S2��34S8������У�����ԭ������Ϊ0.2NA
��12g���ʯ���еĹ��ۼ���Ϊ2NA
��10mL��������Ϊ98%��H2SO4����ˮ��100mL��H2SO4��������������9.8%
�ݺ�0.2NA�������ӵ�Na2O2��ˮ��Ӧʱ��ת��0.2mol����
��11.2LCl2ͨ����������������Һ�г�ַ�Ӧ��ת�Ƶĵ���������0.5NA
��1L��NA��NH3 H2O�İ�ˮ����Ũ��Ϊ1mol
H2O�İ�ˮ����Ũ��Ϊ1mol L-1
L-1
A. �ڢۢ� B. �ۢܢ� C. �ܢޢ� D. �٢ڢ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2017�����ʡ����2�·�ģ���������ۻ�ѧ�Ծ��������棩 ���ͣ�ѡ����
���ֶ�����Ԫ��A��B��C��D��E��F��ԭ������������������A��E��B��Fͬ���壬E��Fͬ���ڣ�D�ĺ˵������F��������������2����B���������������۵Ĵ�����Ϊ0�������µ���A��E��״̬��ͬ�������ж���ȷ����
A. A��D����Ԫ���γɵĻ�������ֻ���м��Լ�
B. A��C��D����Ԫ���γɵĻ�����һ���ǹ��ۻ��������Һһ��������
C. ԭ�Ӱ뾶�ɴ�С��˳����F��E��C��D
D. ����������Ӧˮ����������ǿ��Ԫ����C
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com