
 ���ñ�NaOH��Һ�ζ�δ֪Ũ�ȵ����ᣬ�÷�̪��ָʾ�������в����лᵼ��ʵ����ƫ�͵���
���ñ�NaOH��Һ�ζ�δ֪Ũ�ȵ����ᣬ�÷�̪��ָʾ�������в����лᵼ��ʵ����ƫ�͵���

 ����ѧ���ʱѧ����ϵ�д�
����ѧ���ʱѧ����ϵ�д� �ʰ�Ӣ��ͬ����ϰ��ϵ�д�
�ʰ�Ӣ��ͬ����ϰ��ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ������人�����ص�ѧУ�߶���ѧ����ĩͳ����ѧ������������ ���ͣ������
��12�֣���Ҫ��ش���������
��1���ñ�NaOH��Һ�ζ�δ֪Ũ�ȵ����ᣬ�÷�̪��ָʾ�������в����лᵼ��ʵ����ƫ�͵��� (����� )
�ټ�ʽ�ζ���������ˮϴ����û���ñ�Һ��ϴ
����ƿ������ˮϴ����û���ô���Һ��ϴ
������ʽ�ζ��ܼӴ���Һʱ����������ˮϴ����ĵζ���δ�ô���Һ��ϴ
�ܵζ�ǰ�ζ��ܼ�������ݣ��ζ���������ʧ
���յ����ʱ���ӣ���������������ȷ
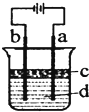
��2��ij����С��ͬѧ����ͼ��װ�ý���ʵ�飬�Դ��������⣺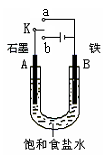
������ʼʱ����K��a���ӣ����������绯ѧ��ʴ�е� ��ʴ��
������ʼʱ����K��b���ӣ����ܷ�Ӧ�����ӷ���ʽΪ ��
��3��������,���ȡ0.1mol/L HA��Һ��0.1mol/L NaOH��Һ��������(���Ի�Ϻ���Һ����ı仯),��û����Һ��pH=8��������Һ��������ʽ�ľ�ȷ����������������֣���c(OH��)��c(HA)�� ___________ mol/L��
��4����Cl����Al3����HSO4����K����HS�����������У�ֻ��ˮ�ⲻ�ܵ���������� ��ֻ�ܵ��벻��ˮ��������� �����ܵ�������ˮ��������� ��д����ˮ�����ӵ�ˮ�����ӷ���ʽ �� ��
��5����֪25��ʱ��Mg(OH)2���ܶȻ�����Ksp = 5.6��10��12�����ij��Һ��pH = 13������¶�����Һ�е�c(Mg2+) = ____________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ�꽭��ʡ�߰���ѧ�߶���ѧ�����п��Ի�ѧ�Ծ����������� ���ͣ������
��18�֣���Ҫ��ش���������
��1���ñ�NaOH��Һ�ζ�δ֪Ũ�ȵ����ᣬ�÷�̪��ָʾ�������в����лᵼ��ʵ����ƫ�͵��� (����� )
�ټ�ʽ�ζ���������ˮϴ����û���ñ�Һ��ϴ
������ʽ�ζ��ܼӴ���Һʱ����������ˮϴ����ĵζ���δ�ô���Һ��ϴ
����ƿ������ˮϴ����û���ô���Һ��ϴ
�ܵζ�ǰ�ζ��ܼ�������ݣ��ζ���������ʧ
���յ����ʱ���ӣ���������������ȷ
��2��ij����С��ͬѧ����ͼ��װ�ý���ʵ�飬�Դ��������⣺
������ʼʱ����K��a���ӣ����������绯ѧ��ʴ�е� ��ʴ��
������ʼʱ����K��b���ӣ����ܷ�Ӧ�����ӷ���ʽΪ ��
��3����֪��Ǧ�����ܵĻ�ѧ����ʽΪ��Pb+PbO2 +2H2SO4 2PbSO4+2H2O
2PbSO4+2H2O
��Ǧ�����ڷŵ�ʱ������ӦΪ ��
��Ǧ�����ڳ��ʱ������ӦΪ ��
�����Ǧ�����ڷŵ�ʱ��·����2mol����ת��ʱ������H2SO4 mol��
��4��������,���ȡ0.1mol/L HA��Һ��0.1mol/L NaOH��Һ��������(���Ի�Ϻ���Һ����ı仯),��û����Һ��pH=8��������Һ��������ʽ�ľ�ȷ����������������֣���
c(OH��)��c(HA)�� ___________ mol/L��
��5����Cl����Al3����HSO4����K����HS�����������У�ֻ��ˮ�ⲻ�ܵ���������� ��ֻ�ܵ��벻��ˮ��������� �����ܵ�������ˮ��������� ��д����ˮ�����ӵ�ˮ�����ӷ���ʽ �� ��
��6����֪25��ʱ��Mg(OH)2���ܶȻ�����Ksp = 5.6��10��12�����ij��Һ��pH = 13������¶�����Һ�е�c(Mg2+) = ____________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013������人�����ص�ѧУ�߶���ѧ����ĩͳ����ѧ���������棩 ���ͣ������
��12�֣���Ҫ��ش���������
��1���ñ�NaOH��Һ�ζ�δ֪Ũ�ȵ����ᣬ�÷�̪��ָʾ�������в����лᵼ��ʵ����ƫ�͵��� (����� )
�ټ�ʽ�ζ���������ˮϴ����û���ñ�Һ��ϴ
����ƿ������ˮϴ����û���ô���Һ��ϴ
������ʽ�ζ��ܼӴ���Һʱ����������ˮϴ����ĵζ���δ�ô���Һ��ϴ
�ܵζ�ǰ�ζ��ܼ�������ݣ��ζ���������ʧ
���յ����ʱ���ӣ���������������ȷ
��2��ij����С��ͬѧ����ͼ��װ�ý���ʵ�飬�Դ��������⣺

 ������ʼʱ����K��a���ӣ����������绯ѧ��ʴ�е�
��ʴ��
������ʼʱ����K��a���ӣ����������绯ѧ��ʴ�е�
��ʴ��
������ʼʱ����K��b���ӣ����ܷ�Ӧ�����ӷ���ʽΪ ��
��3��������,���ȡ0.1mol/L HA��Һ��0.1mol/L NaOH��Һ��������(���Ի�Ϻ���Һ����ı仯),��û����Һ��pH=8��������Һ��������ʽ�ľ�ȷ����������������֣���c(OH��)��c(HA)�� ___________ mol/L��
��4����Cl����Al3����HSO4����K����HS�����������У�ֻ��ˮ�ⲻ�ܵ���������� ��ֻ�ܵ��벻��ˮ��������� �����ܵ�������ˮ��������� ��д����ˮ�����ӵ�ˮ�����ӷ���ʽ �� ��
��5����֪25��ʱ��Mg(OH)2���ܶȻ�����Ksp = 5.6��10��12�����ij��Һ��pH = 13������¶�����Һ�е�c(Mg2+) = ____________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013�콭��ʡ�߶���ѧ�����п��Ի�ѧ�Ծ��������棩 ���ͣ������
��18�֣���Ҫ��ش���������
��1���ñ�NaOH��Һ�ζ�δ֪Ũ�ȵ����ᣬ�÷�̪��ָʾ�������в����лᵼ��ʵ����ƫ�͵��� (����� )
�ټ�ʽ�ζ���������ˮϴ����û���ñ�Һ��ϴ
������ʽ�ζ��ܼӴ���Һʱ����������ˮϴ����ĵζ���δ�ô���Һ��ϴ
����ƿ������ˮϴ����û���ô���Һ��ϴ
�ܵζ�ǰ�ζ��ܼ�������ݣ��ζ���������ʧ
���յ����ʱ���ӣ���������������ȷ
��2��ij����С��ͬѧ����ͼ��װ�ý���ʵ�飬�Դ��������⣺
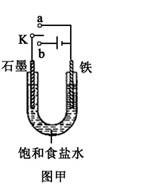
������ʼʱ����K��a���ӣ����������绯ѧ��ʴ�е� ��ʴ��
������ʼʱ����K��b���ӣ����ܷ�Ӧ�����ӷ���ʽΪ ��
��3����֪��Ǧ�����ܵĻ�ѧ����ʽΪ��Pb+PbO2 +2H2SO4 2PbSO4+2H2O
2PbSO4+2H2O
��Ǧ�����ڷŵ�ʱ������ӦΪ ��
��Ǧ�����ڳ��ʱ������ӦΪ ��
�����Ǧ�����ڷŵ�ʱ��·����2mol����ת��ʱ������H2SO4 mol��
��4��������,���ȡ0.1mol/L HA��Һ��0.1mol/L NaOH��Һ��������(���Ի�Ϻ���Һ����ı仯),��û����Һ��pH=8��������Һ��������ʽ�ľ�ȷ����������������֣���
c(OH��)��c(HA)�� ___________ mol/L��
��5����Cl����Al3����HSO4����K����HS�����������У�ֻ��ˮ�ⲻ�ܵ���������� ��ֻ�ܵ��벻��ˮ��������� �����ܵ�������ˮ��������� ��д����ˮ�����ӵ�ˮ�����ӷ���ʽ �� ��
��6����֪25��ʱ��Mg(OH)2���ܶȻ�����Ksp = 5.6��10��12�����ij��Һ��pH = 13������¶�����Һ�е�c(Mg2+) = ____________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��Ҫ��ش���������
��1���ñ�NaOH��Һ�ζ�δ֪Ũ�ȵ����ᣬ�÷�̪��ָʾ�������в����лᵼ��ʵ����ƫ�͵��� (����� )
�ټ�ʽ�ζ���������ˮϴ����û���ñ�Һ��ϴ ����ƿ������ˮϴ����û���ô���Һ��ϴ
������ʽ�ζ��ܼӴ���Һʱ����������ˮϴ����ĵζ���δ�ô���Һ��ϴ
�ܵζ�ǰ�ζ��ܼ�������ݣ��ζ���������ʧ ���յ����ʱ���ӣ���������������ȷ
��2��ij����С��ͬѧ����ͼ��װ�ý���ʵ�飬�Դ��������⣺
 ������ʼʱ����K��a���ӣ����������绯ѧ��ʴ�е� ��ʴ��
������ʼʱ����K��a���ӣ����������绯ѧ��ʴ�е� ��ʴ��
������ʼʱ����K��b���ӣ����ܷ�Ӧ�����ӷ���ʽΪ ��
��3��������,���ȡ0.1mol/L HA��Һ��0.1mol/L NaOH��Һ��������(���Ի�Ϻ���Һ����ı仯),��û����Һ��pH=8��������Һ��������ʽ�ľ�ȷ����������������֣���c(OH��)��c(HA)�� ___________ mol/L��
��4����Cl����Al3����HSO4����K����HS�����������У�ֻ��ˮ�ⲻ�ܵ���������� ��ֻ�ܵ��벻��ˮ��������� �����ܵ�������ˮ��������� ��д����ˮ�����ӵ�ˮ�����ӷ���ʽ �� ��
��5����֪25��ʱ��Mg(OH)2���ܶȻ�����Ksp = 5.6��10��12�����ij��Һ��pH = 13������¶�����Һ�е�c(Mg2+) = ____________________��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com