
����Ŀ��һ���ܱ��������м���һ�����ɻ����ĸ���(��Ȳ���)�������ֳ������֣�����߳���8molN2,�ұ߳���CO��CO2�Ļ�����干64gʱ�����崦����ͼλ��(�����¶Ȳ���),����˵����ȷ����(�� ��)
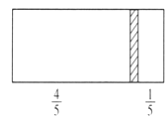
A. �ұ�CO��CO2������֮��Ϊ3:1
B. �Ҳ�CO������Ϊ14g
C. �Ҳ������ܶ�����ͬ�����������ܶȵ�2��
D. ���ı��ұ�CO��CO2�ij�������ʹ���崦�ھ����Ҷ�1/3���������¶Ȳ��䣬��ǰ�����γ�����������ڵ�ѹǿ֮��Ϊ6: 5
���𰸡�A
��������
�������������¶ȡ�ѹǿ��ͬ����ͬ�����£����֮�ȵ������ʵ���֮�ȣ��������֮��Ϊ4:1���������������ʵ���֮��Ϊ4:1�������Ҳ��������ʵ���=8mol/4=2mol��CO��CO2����Ϊ64g����CO�����ʵ���Ϊxmol���������̼���ʵ���Ϊ(2x)mol��28xg+44(2x)g=64g��x=1.5mol����CO�����ʵ���Ϊ1.5mol��������̼�����ʵ���Ϊ0.5mol��
A.��������ʵ���֮�ȵ����������֮�ȣ������ұ�CO��CO2������֮��Ϊ1.5mol:0.5mol=3:1����A��ȷ��
B.m(CO)=nM=1.5mol��28g/mol=42g����B����
C.��ͬ�����������ܶ�֮�ȵ�����Ħ������֮�ȣ��ұ�����ƽ��Ħ������=64g/2mol=32g/mol��������Ħ��������ȣ����Ի�������������ܶ�֮��Ϊ1:1����C����
D.���ı��ұ�CO��CO2�ij�������ʹ���崦�ھ����Ҷ�1/3���������ҿռ����֮��Ϊ2:1�������������ʵ���֮��Ϊ2:1�����������̼��CO���ʵ���Ϊ4mol����ͬ��������������ʵ���֮�ȵ�����ѹǿ֮����������ѹǿ֮��Ϊ(8+2)mol:(8+4)mol=5:6����D����ѡA��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��CoC2O4���Ʊ������ܵ�ԭ�ϡ����ú��ܷ��ϣ���Ҫ�ɷ�ΪCo2O3��������Fe2O3��Al2O3��CaO��MgO��̼���л���ȣ���ȡCoC2O4�Ĺ����������£�
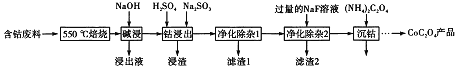
��1����500����������Ŀ����_______��
��2��������Һ������Ҫ�ɷ���_______��
��3�����ܽ�����������Co3+ת��ΪCo2+����Ӧ�����ӷ���ʽΪ_________��
��4������������1�������У�����40��50�� ����H2O2��Һ����Ŀ����_____________��
�������ӷ���ʽ��ʾ������������80��85��������Na2CO3��Һ����pH��5��������I������Ҫ�ɷ���___________��
��5������������2���ɽ��ơ�þ����ת��Ϊ�������˳�ȥ����������Һ��c(Ca2+)=1.0��10-5mol/L����c(Mg2+)Ϊ________����֪Ksp(MgF2)=7.35��10-11���� Ksp(CaF2)=1.05��10-10��
��6��Ϊ�ⶨ�Ƶõ�CoC2O4��Ʒ�Ĵ��ȣ��ֳ�ȡ1.00g��Ʒ���������ʵ��Լ�ת��Ϊ�����[(NH4��2C2O4����Һ�����ù���ϡ�����ữ����0.1000mol/L���������Һ�ζ�����_________ʱ�����ﵽ�ζ��յ㣬����ȥ���������Һ26.00mL���ò�Ʒ�Ĵ���Ϊ_______________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�������Ȼ�ѧ����ʽ��ȷ����
A�������ȼ����Ϊ890.3 kJ��mol-1�������ȼ�յ��Ȼ�ѧ����ʽ�ɱ�ʾΪ
CH4(g)��2O2(g)===CO2(g)��2H2O(g) ��H����890.3 kJ��mol-1
B��500 �桢30 MPa�£���0.5 mol N2��1.5 mol H2�����ܱ������г�ַ�Ӧ����NH3(g)������19.3 kJ�����Ȼ�ѧ����ʽΪN2(g)��3H2(g)![]() 2NH3(g) ��H����38.6 kJ��mol-1
2NH3(g) ��H����38.6 kJ��mol-1
C����֪��101 kPa�£�1 g H2ȼ������ˮ�����ų�121 kJ���������Ȼ�ѧ����ʽΪ
H2(g)��![]() O2(g)===H2O(g) ��H����242 kJ��mol-1
O2(g)===H2O(g) ��H����242 kJ��mol-1
D��25 �棬101 kPaʱ��ǿ����ǿ���ϡ��Һ�����кͷ�Ӧ���к���Ϊ57.3 kJ��mol-1����ʾϡ����������������Һ��Ӧ���к��ȵ��Ȼ�ѧ����ʽΪH2SO4(aq)��2KOH(aq)=K2SO4(aq)��2H2O(l) ��H����114.6 kJ��mol-1
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ij��ѧС��Ϊ�ⶨһ��������ijͭ���������ͭ���������������������ʵ�鷽����
��������ͭ�������![]() �ⶨ������������
�ⶨ������������
��������ͭ�������![]() �ⶨʣ����������
�ⶨʣ����������
�����й��ж��в���ȷ����
A. ��ҺA��B�����������������������Һ
B. ��ҺA��B������ѡ��ϡ����
C. ����ҺBѡ���Ȼ�����Һ����ʵ������ʵ��
D. ʵ�鷽����������ʵʩ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���ס���ʵ��С���������ʵ��װ�÷ֱ��Ʊ�SnCl4��Ư�ۡ�
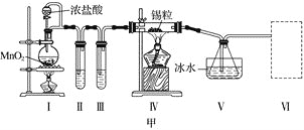

(1)��֪��a.�������۵�231 �棬��ѧ�������������ƣ�
b����������������ڽ�������Ӧ����SnCl4��SnCl4�ķе�114 ����
c��SnCl2��SnCl4����ˮ�⣬�������л��ܼ�����Sn2���ױ�����������ͼ��װ�ûش�
���Թܢ��е��Լ���____________________�����е��Լ���________________________��
�ڢ���װ�����ѡ��________(����ĸ)��

�ۢ�װ���з�Ӧ�����ӷ���ʽ��____________________________________________��
��ʵ�����������������װ�â���δ��Ӧ���MnO2����Ҫ�IJ���������______________��
(2)��֪����Cl2�볱ʪ����ʯ�ҷ�Ӧ�Ƿ��ȷ�Ӧ�����¶Ƚϸ�ʱCl2�볱ʪCa(OH)2�ķ�ӦΪ6Cl2��6Ca(OH)2=Ca(ClO3)2��5CaCl2��6H2O��
����ͼ��װ�ûش𣺴�ʵ������Ca(ClO)2���ʽϵ͵�������
��________________________________________________________________________��
��________________________________________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
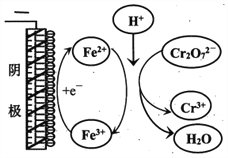
����Ŀ���о�+6�۸��β�ͬ��������������ʽ�������ԣ�ijС��ͬѧ��������ʵ�飺
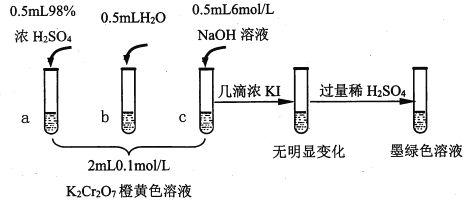
��֪��Cr2O72- (��ɫ)+H2O![]() 2CrO42-(��ɫ)+2H+ ��H= +13.8 kJ/mol��+6�۸�����һ�������¿ɱ���ԭΪCr3+��Cr3+��ˮ��Һ��Ϊ��ɫ��
2CrO42-(��ɫ)+2H+ ��H= +13.8 kJ/mol��+6�۸�����һ�������¿ɱ���ԭΪCr3+��Cr3+��ˮ��Һ��Ϊ��ɫ��
��1���Թ�c��b�Աȣ��Ʋ��Թ�c��������________��
��2���Թ�a��b�Աȣ�a����Һ��ɫ�������Ϊ�¶�Ҳ��Ӱ��ƽ����ƶ�����ɫ���һ����c(H+)����Ӱ��Ľ��������Ϊ��ɫ����һ����c(H+)�����ƽ���Ӱ�졣����Ϊ�Ƿ���Ҫ�����ʵ��֤����____�����ǡ�����������_________________________________��
��3���Ա��Թ�a��b��c��ʵ�����õ��Ľ�����________________��
��4���Թ�c�����μ�KI��Һ������ϡH2SO4��������ͼ��ʵ�����ó��Ľ�����_______��д���˹�����������ԭ��Ӧ�����ӷ���ʽ________________��
��5��С��ͬѧ�õ�ⷨ������Cr2O72-��ˮ��̽����ͬ���ضԺ�Cr2O72-��ˮ������Ӱ�죬������±���ʾ��Cr2O72-����ʼŨ�ȣ��������ѹ�����ʱ�����ͬ����
ʵ�� | �� | �� | �� | �� |
�Ƿ����Fe2(SO4)3 | �� | �� | ����5g | �� |
�Ƿ����H2SO4 | �� | ����1mL | ����1mL | ����1mL |
�缫���� | ����������Ϊʯī | ����������Ϊʯī | ����������Ϊʯī | ����Ϊʯī������Ϊ�� |
Cr2O72-��ȥ����/% | 0.922 | 12.7 | 20.8 | 57.3 |
��ʵ������Cr2O72-�ŵ�ĵ缫��Ӧʽ��________________��
��ʵ������Fe3+ȥ��Cr2O72-�Ļ�����ͼ��ʾ����ϴ˻���������ʵ��iv��Cr2O72-ȥ������߽϶��ԭ��_______________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���л���F��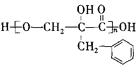 ��Ϊһ�ָ߷�����֬����ϳ�·�����£�
��Ϊһ�ָ߷�����֬����ϳ�·�����£�
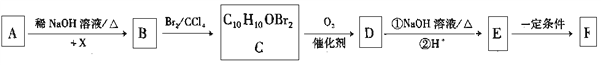
��֪����AΪ����ȩ��ͬϵ���������������Է�������Ϊ134��
��
��ش��������⣺
��1��X�Ļ�ѧ������_________________��
��2��E����F�ķ�Ӧ����Ϊ_________________��
��3��D�Ľṹ��ʽΪ_________________��
��4����B����C�Ļ�ѧ����ʽΪ_________________��
��5�������廯����Y��D��ͬϵ�Y��ͬ���칹�����뱥��Na2CO3��Һ��Ӧ�ų����壬������ֻ��1���������˴Ź���������ʾ��5�ֲ�ͬ��ѧ�������⣬��ֵ�����Ϊ6:2:2:1:1��д�����ַ���Ҫ���Y�Ľṹ��ʽ___________��__________��
��6��д���Լ�ȩ����ȩ���Ҷ���Ϊ��Ҫԭ�Ϻϳ����������۾��߷��Ӳ��ϡ��ۼ���ϩ���������� ���ĺϳ�·�ߣ����Լ���ѡ����_________________��
���ĺϳ�·�ߣ����Լ���ѡ����_________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
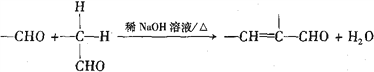
����Ŀ���㶹���������з�����ζ������ҩ�е�һ����Ҫ�Ļ��Գɷ֣���Ҫ�ֲ���ɡ�οơ����ơ��տơ�ܿ��ơ��ѿơ�����ơ����Ƶ�ֲ���С�������X��һ���㶹������������������ϣ���ϳ�·�����£�
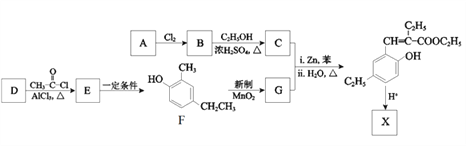
��֪��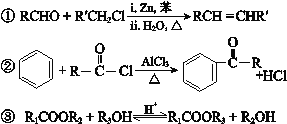
��1����֪A�ķ���ʽΪC4H8O2��A���������ŵ�������______________��
��2��B����C��Ӧ�Ļ�ѧ����ʽ��______________________________________________��
��3��G�Ľṹ��ʽ��__________________________��
��4��D�ķ���ʽ��C7H8O����F��Ϊͬϵ���D�Ľṹ��ʽ��___________________��
��5��E���Ծ��ಽ��Ӧ�ϳ�F����д��һ�����ܵĺϳ�·��_________���ýṹ��ʽ��ʾ�л���ü�ͷ��ʾת����ϵ����ͷ��ע���Լ��ͷ�Ӧ��������
��6��X�ķ���ʽ��C13H14O2��X������NaOH��Һ���ȵĻ�ѧ����ʽ��________________
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��A��Al2O3��Fe3O4��Al��Cu�е�ij���ַ�ĩ��϶��ɣ���Ƴɷַ����������£����з�������ȷ���ǣ� ��
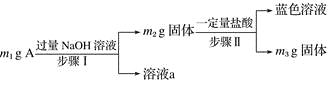
A. ��m1>m2ʱ����Һa��������ֻ��1��
B. ������ɫ��Һ�����ӷ���ʽ��Cu��2Fe3��===Cu2����2Fe2��
C. Ҫȷ����������Ƿ�Al����ȡA��������ϡHCl
D. ��m2��m3��2.96 g��Fe3O4����������Ϊ2.32 g
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com