
��������Դ�����ϴ������ǿɳ�����չ����Ҫ���档
��1����̼��������ֱ�Ӻϳ��Ҵ�ȼ���ѽ�����ģ���������ȡ��CO��H2Ϊԭ�Ϻϳ���������ѧ��Ӧ����ʽ��2CO(g)+4H2(g) CH3CH2OH(g)+H2O(g)�����ܱ������г���10 mol CO��20mol H2���ڴ��������·�Ӧ�����Ҵ���CO��ת����(��)���¶ȡ�ѹǿ�Ĺ�ϵ����ͼ��ʾ��
CH3CH2OH(g)+H2O(g)�����ܱ������г���10 mol CO��20mol H2���ڴ��������·�Ӧ�����Ҵ���CO��ת����(��)���¶ȡ�ѹǿ�Ĺ�ϵ����ͼ��ʾ��
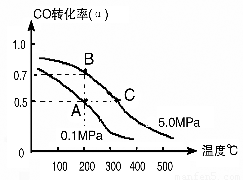
����A��B�����ʾ��ijʱ�̴ﵽ��ƽ��״̬����ʱ��A��ʱ���������Ϊ10L������¶��µ�ƽ�ⳣ����K��??????? ��
����A��C���㶼��ʾ�ﵽ��ƽ��״̬�����Է�Ӧ��ʼ����ƽ��״̬�����ʱ��tA???? tC������ڡ�����С�ڡ����ڡ�����
�۹�ҵ�ϻ����Բ�ȡ��CO2��H2Ϊԭ�Ϻϳ��Ҵ������Ҹ�����ѧ�������Ƴ磬��������ͬ�����£���CO��ȡCH3CH2OH��ƽ�ⳣ��ԶԶ������CO2��ȡCH3CH2OH ��ƽ�ⳣ�������Ʋ⻯ѧ�������Ͽ���CO2��ȡCH3CH2OH���ŵ���Ҫ�ǣ�???????????????????????????????????? ��
��2��Ŀǰ��ҵ��Ҳ������CO2�������״���һ�������·�����ӦCO2(g)��3H2(g) CH3OH(g)��H2O(g)������6mol CO2��8 mol H2����2L���ܱ������У����H2�����ʵ�����ʱ��仯����������ͼ��ʾ��ʵ�ߣ���
CH3OH(g)��H2O(g)������6mol CO2��8 mol H2����2L���ܱ������У����H2�����ʵ�����ʱ��仯����������ͼ��ʾ��ʵ�ߣ���
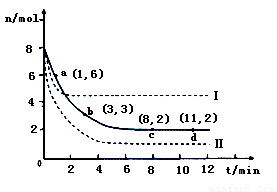
�����ڴ����ͼ�л���״������ʵ�����ʱ��仯���ߡ�
�ڽ��ı�ijһʵ�������ٽ�������ʵ�飬���H2�����ʵ�����ʱ��仯��ͼ��������ʾ������I��Ӧ��ʵ�������ı���????????? ? �����ߢ��Ӧ��ʵ�������ı���??????? ��
��3��Hg��ˮ����Ⱦ���ؽ���Ԫ��֮һ��ˮ��Һ�ж��۹�����Ҫ������̬��Cl����OH����Ũ�ȹ�ϵ����ͼ��ʾ��ͼ�е����ʻ�����ֻ��Hg(OH)2Ϊ�����pCl=��1gc(Cl��)��
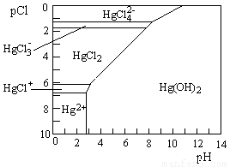
������˵������ȷ����????? ��
A��Ϊ�˷�ֹHg2��ˮ��������Hg(NO3)2��ҺʱӦ��Hg(NO3)2��������Ũ�������ϡ��
B����c(C1��) ��10��1 mol��L��1ʱ����Ԫ��һ��ȫ����HgCl42����ʽ����
C��HgCl2��һ��������ʣ�����뷽��ʽ�ǣ�HgCl2��HgCl�� + C1��
D������ҺpH������4��pCl��2�ı���6ʱ����ʹHgCl2ת��ΪHg(OH)2
��HgCl2�ֳ������������۵�549K���������������侧����????? ��������ͣ���
��1����K=0.25 ��2�֣�?? �ڴ��ڣ�2�֣� ��ԭ���á����Լ�������ЧӦ�ȣ�2�֣�
��2���٣�2����
��? ���£� ��ѹ����1�֣�
��3����AD??? �ڷ��Ӿ���
��������
�����������1����????? 2CO(g)+4H2(g) CH3CH2OH(g)+H2O(g)
CH3CH2OH(g)+H2O(g)
��ʼŨ�ȣ�mol/L�� 1????? 2???????????? 0?????????? 0
ת��Ũ�ȣ�mol/L��0.5??? 1.0??????????? 0.25?????? 0.25
ƽ��Ũ�ȣ�mol/L��0.5??? 1.0??????????? 0.25????? 0.25
������ѧƽ�ⳣ������һ�������£������淴Ӧ�ﵽƽ��״̬ʱ��������Ũ�ȵ���֮���ͷ�Ӧ��Ũ�ȵ���֮���ı�ֵ��֪�����¶��·�Ӧ��ƽ�ⳣ��K��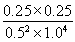 ��0.25��
��0.25��
�ڸ���ͼ���֪��C���¶Ⱥ�ѹǿ������A���¶Ⱥ�ѹǿ������C�㷴Ӧ���ʿ죬�ﵽƽ���ʱ���١�
�����ڵ�����CO2�ĺ������������Ը÷������ŵ���ԭ���á����Լ�������ЧӦ��
��2���ٸ���ͼ���֪ƽ��ʱ���������ʵ�����2mol���������������ʵ�����8mol��2mol��6mol�����Ը��ݷ���ʽ��֪ƽ��ʱ���ɼ״������ʵ�����2mol�����ͼ��ɱ�ʾΪ���𰸡�
������I��ԭ������ȴﵽƽ���ʱ����٣�˵����Ӧ���ʿ졣��ƽ��ʱ���������ʵ������ӣ�˵��ƽ�����淴Ӧ�����ƶ�����������Ӧ�������С�Ŀ��淴Ӧ�����Ըı������ֻ���������¶ȣ�������Ӧ�Ƿ��ȷ�Ӧ�����ߢ���ԭ������ȴﵽƽ���ʱ����٣�˵����Ӧ���ʿ졣��ƽ��ʱ���������ʵ������٣���˵��ƽ��������Ӧ�����ƶ�����������Ӧ�������С�ķ��ȵĿ��淴Ӧ�����Ըı������ֻ��������ѹǿ��
��3����A��Hg2��ˮ����Һ�����ԣ�����Ϊ�˷�ֹHg2��ˮ��������Hg(NO3)2��ҺʱӦ��Hg(NO3)2��������Ũ�������ϡ����A��ȷ��B����c(C1��) ��10��1 mol��L��1��pCl��1ʱ����Ԫ����Ҫ����HgCl42����ʽ������B����ȷ��C��HgCl2��һ��������ʣ�����뷽��ʽ�ǣ�2HgCl2 HgCl�� + HgCl3����C����ȷ��D������ͼ���֪����ҺpH������4��pCl��2�ı���6ʱ����ʹHgCl2ת��ΪHg(OH)2��D��ȷ����ѡAD��
HgCl�� + HgCl3����C����ȷ��D������ͼ���֪����ҺpH������4��pCl��2�ı���6ʱ����ʹHgCl2ת��ΪHg(OH)2��D��ȷ����ѡAD��
��HgCl2�ֳ������������۵�549K����������������˵�����������Ӿ��塣
���㣺����ƽ�ⳣ���ļ��㡢���������ƽ��״̬��Ӱ���Լ��ܽ�ƽ����й�Ӧ�����ж�



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com