��1���й��Ŵ��Ĵ���֮һ--�ڻ�ҩ�����ı�ը��ӦΪ��
2KNO
3+3C+S
A+N
2��+3CO
2��������ƽ��
�ٳ�S�⣬����Ԫ�صĵ縺�ԴӴ�С����Ϊ
O��N��C��K
O��N��C��K
��
�����������У�A�ľ�������Ϊ
���Ӿ���
���Ӿ���
�������Թ��ۼ��ķ��ӵ�����ԭ�ӹ���ӻ�����Ϊ
sp�ӻ�
sp�ӻ�
��
����֪CN
-��N
2Ϊ�ȵ����壬����HCN�����ЦҼ���м���Ŀ֮��Ϊ
1��1
1��1
��
��2��ԭ������С��36��Ԫ��Q��T�������ڱ��мȴ���ͬһ������λ��ͬһ�壬��ԭ������T��Q��2��T�Ļ�̬ԭ����Χ���ӣ��۵��ӣ��Ų�Ϊ
3d84s2
3d84s2
��Q
2+��δ�ɶԵ�������
4
4
��
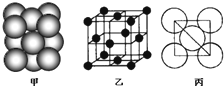
��3����ij�������ʾ�����ԭ�ӵĶѻ���ʽ��ͼ����ʾ���侧��������ͼ����ʾ��ԭ��֮���λ�ù�ϵ��ƽ��ͼ��ͼ����ʾ�����и�ԭ�ӵ���λ��Ϊ
12
12
���õ��ʾ�����ԭ�ӵĶѻ���ʽΪ���ֻ����ѻ���ʽ�е�
ͭ��
ͭ��
��
��4����CrCl
3��ˮ��Һ�У�һ�������´������Ϊ[CrCl
n��H
2O��
6-n]
x+��n��x��Ϊ���������������ӣ�����ͨ�������ӽ�����֬��R-H�����ɷ������ӽ�����Ӧ��[CrCl
n��H
2O��
6-n]
x++xR-H-��R
x[CrCl
n��H
2O��
6-n]
x++xH
+����������H
+���к͵ζ����������x��n��ȷ�������ӵ���ɣ�����0.0015mol[CrCl
n��H
2O��
6-n]
x+����Һ����R-H��ȫ�������к����ɵ�H
+��Ũ��Ϊ0.1200mol?L
-1NaOH��Һ25.00mL����֪�������ӵĻ�ѧʽΪ
[CrCl��H2O��5]2+
[CrCl��H2O��5]2+
���������ӵ���λ��Ϊ
6
6
��




 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
 ��1���й��Ŵ��Ĵ���֮һ--�ڻ�ҩ�����ı�ը��ӦΪ��2KNO3+3C+S
��1���й��Ŵ��Ĵ���֮һ--�ڻ�ҩ�����ı�ը��ӦΪ��2KNO3+3C+S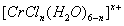 ��n��x��Ϊ���������������ӣ�����ͨ�������ӽ�����֬��R-H�����ɷ������ӽ�����Ӧ��
��n��x��Ϊ���������������ӣ�����ͨ�������ӽ�����֬��R-H�����ɷ������ӽ�����Ӧ��  Rx[CrCl3(H2O)6-n]x+ +xH+
Rx[CrCl3(H2O)6-n]x+ +xH+