
题目列表(包括答案和解析)
某同学取一定量淀粉进行水解实验,其程序如下所示:

请回答下列问题:
(1)所加入的试剂A ,B ,C ;
(2)简答加B的原因: ;
(3)由此可知淀粉是 (填部分、完全、没有)水解。
| |||||||||||||||
| 物 质 | Fe(OH)2 | Cu(OH)2 | Fe(OH)3 |
| 完全沉淀时的pH范围 | ≥9.6 | ≥6.4 | 3~4 |
| Ⅰ |
| Ⅱ |
| Ⅲ |

氧化铜有多种用途,如用作玻璃着色剂,油类脱硫剂等,为获得纯净的氧化铜并探究其性质,某同学查找了溶度积数据并通过计算得到有关信息(见下表),用工业硫酸铜(含硫酸亚铁等杂质)进行如下实验:
| 物 质 | Fe(OH)2 | Cu(OH)2 | Fe(OH)3 |
| 完全沉淀时的pH范围 | ≥9.6 | ≥6.4 | 3~4 |
㈠制备氧化铜
![]()
⑴步骤Ⅰ的操作是加入水和少量硫酸溶解样品并过滤,目的是除去不溶性杂质,这一步骤中加酸的作用是 。
⑵步骤Ⅱ的操作是:滴加H2O2溶液,稍加热;待反应完全后,慢慢加入Cu2(OH)2CO3粉末,搅拌,以控制溶液pH=3.5;加热煮沸一段时间,过滤,用稀硫酸酸化滤液至pH=1。
①这一步骤的目的是 ,
②写出加入H2O2溶液时发生反应的离子方程式
③控制溶液pH=3.5的目的是 ,
⑶步骤Ⅲ的目的是得到CuSO4·5H2O晶体,操作是 ,水浴加热烘干所得固体。水浴加热的特点是 。
㈡探究氧化铜性质
⑴取A、B两支试管,往A中先加入适量CuO粉末,再分别向A和B中加入等体积的3%H2O2溶液,只观察到A中有大量气泡,结论是 。
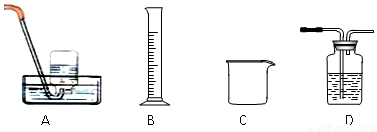
⑵为探究试管A中反应的速率,收集气体并测定其体积必需的实验仪器或装置为 。(填写序号)
氧化铜有多种用途,如用作玻璃着色剂,油类脱硫剂等,为获得纯净的氧化铜并探究其性质,某同学查找了溶度积数据并通过计算得到有关信息(见下表),用工业硫酸铜(含硫酸亚铁等杂质)进行如下实验:
|
物 质 |
Fe(OH)2 |
Cu(OH)2 |
Fe(OH)3 |
|
完全沉淀时的pH范围 |
≥9.6 |
≥6.4 |
3~4 |
㈠制备氧化铜
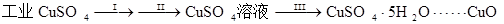
⑴步骤Ⅰ的操作是加入水和少量硫酸溶解样品并过滤,目的是除去不溶性杂质,这一步骤中加酸的作用是 。
⑵步骤Ⅱ的操作是:滴加H2O2溶液,稍加热;待反应完全后,慢慢加入Cu2(OH)2CO3粉末,搅拌,以控制溶液pH=3.5;加热煮沸一段时间,过滤,用稀硫酸酸化滤液至pH=1。
①这一步骤的目的是 ,
②写出加入H2O2溶液时发生反应的离子方程式
③控制溶液pH=3.5的目的是 ,
⑶步骤Ⅲ的目的是得到CuSO4·5H2O晶体,操作是 ,水浴加热烘干所得固体。水浴加热的特点是 。
㈡探究氧化铜性质
⑴取A、B两支试管,往A中先加入适量CuO粉末,再分别向A和B中加入等体积的3%H2O2溶液,只观察到A中有大量气泡,结论是 。
⑵为探究试管A中反应的速率,收集气体并测定其体积必需的实验仪器或装置为 。(填写序号)
湖北省互联网违法和不良信息举报平台 | 网上有害信息举报专区 | 电信诈骗举报专区 | 涉历史虚无主义有害信息举报专区 | 涉企侵权举报专区
违法和不良信息举报电话:027-86699610 举报邮箱:58377363@163.com