
题目列表(包括答案和解析)
将0.2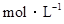 的CH3COONa溶液与0.1
的CH3COONa溶液与0.1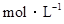 的HCl溶液等体积混合后,溶液中下列微粒的物质的量浓度的关系中,正确的是
的HCl溶液等体积混合后,溶液中下列微粒的物质的量浓度的关系中,正确的是
A.[CH3COO-] = [Cl-] = [H+] > [CH3COOH]
B.[CH3COO-] = [[Cl-] > [CH3COO-] > [H+]
C.[CH3COO-] > [Cl-] > [H+] > [ CH3COOH]
D.[CH3COO-] > [Cl-] > [ CH3COOH] > [H+]
[ ]
将0.2![]() 的CH3COONa溶液与0.1
的CH3COONa溶液与0.1![]() 的HCl溶液等体积混合后,溶液中下列微粒的物质的量浓度的关系中,正确的是
的HCl溶液等体积混合后,溶液中下列微粒的物质的量浓度的关系中,正确的是
A.[CH3COO-] = [Cl-] = [H+]> [CH3COOH]
B.[CH3COO-] = [[Cl-] >[CH3COO-] > [H+]
C.[CH3COO-] > [Cl-] >[H+] > [ CH3COOH]
D.[CH3COO-] > [Cl-] >[ CH3COOH] > [H+]
将0.2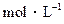 的CH3COONa溶液与0.1
的CH3COONa溶液与0.1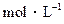 的HCl溶液等体积混合后,溶液中下列微粒的物质的量浓度的关系中,正确的是
的HCl溶液等体积混合后,溶液中下列微粒的物质的量浓度的关系中,正确的是
| A.[CH3COO-] = [Cl-] = [H+] > [CH3COOH] |
| B.[CH3COO-] = [[Cl-] > [CH3COO-] > [H+] |
| C.[CH3COO-] > [Cl-] > [H+] > [ CH3COOH] |
| D.[CH3COO-] > [Cl-] > [ CH3COOH] > [H+] |
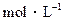 的CH3COONa溶液与0.1
的CH3COONa溶液与0.1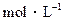 的HCl溶液等体积混合后,溶液中下列微粒的物质的量浓度的关系中,正确的是
的HCl溶液等体积混合后,溶液中下列微粒的物质的量浓度的关系中,正确的是| A.[CH3COO-] = [Cl-] = [H+] > [CH3COOH] |
| B.[CH3COO-] = [[Cl-] > [CH3COO-] > [H+] |
| C.[CH3COO-] > [Cl-] > [H+] > [ CH3COOH] |
| D.[CH3COO-] > [Cl-] > [ CH3COOH] > [H+] |
湖北省互联网违法和不良信息举报平台 | 网上有害信息举报专区 | 电信诈骗举报专区 | 涉历史虚无主义有害信息举报专区 | 涉企侵权举报专区
违法和不良信息举报电话:027-86699610 举报邮箱:58377363@163.com