
题目列表(包括答案和解析)
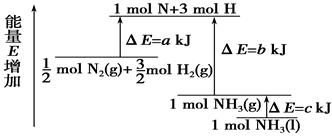
化学反应N2+3H2===2NH3的能量变化如右图所示,该反应的热化学方程式是
A.N2(g)+3H2(g)===2NH3(l) ΔH=2(a-b-c)kJ·mol-
B.N2(g)+3H2(g)===2NH3(g) ΔH=2(b-a)kJ·mol-1
C. 1/2 N2 (g)+3/2H2(g)===NH3(l) ΔH=(b+c-a)kJ·mol-1
D. 1/2 N2(g)+3/2H2(g)===NH3(g) ΔH=(a+b)kJ·mol-1
| A.N2(g)+3H2(g)===2NH3(l) ΔH=2(a-b-c)kJ·mol- |
| B.N2(g)+3H2(g)===2NH3(g) ΔH=2(b-a)kJ·mol-1 |
| C.1/2 N2 (g)+3/2H2(g)===NH3(l) ΔH=(b+c-a)kJ·mol-1 |
| D.1/2 N2(g)+3/2H2(g)===NH3(g) ΔH=(a+b)kJ·mol-1 |
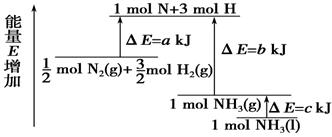
化学反应N2+3H2 = 2NH3的能量变化如右图 所示,该反应的热化学方程式是
所示,该反应的热化学方程式是
A、N2(g)+3H2(g) = 2NH3(l);⊿H = 2(a—b—c)kJ/mol
B、N2(g)+3H2(g) = 2NH3(g) ;⊿H = 2(b—a)kJ/mol
C、![]() N2(g)+
N2(g)+![]() H2(g) = NH3(l) ;⊿H = (b+c—a)kJ/mol
H2(g) = NH3(l) ;⊿H = (b+c—a)kJ/mol
D、![]() N2(g)+
N2(g)+![]() H2(g) =NH3(g) ;⊿H = (a+b)kJ/mol
H2(g) =NH3(g) ;⊿H = (a+b)kJ/mol
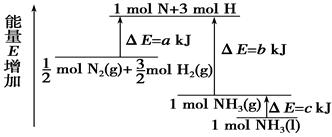
化学反应N2+3H2===2NH3的能量变化如右图所示,该反应的热化学方程式是
| A.N2(g)+3H2(g)===2NH3(l) ΔH=2(a-b-c)kJ·mol- |
| B.N2(g)+3H2(g)===2NH3(g) ΔH=2(b-a)kJ·mol-1 |
| C.1/2 N2 (g)+3/2H2(g)===NH3(l) ΔH=(b+c-a)kJ·mol-1 |
| D.1/2 N2(g)+3/2H2(g)===NH3(g) ΔH=(a+b)kJ·mol-1 |
湖北省互联网违法和不良信息举报平台 | 网上有害信息举报专区 | 电信诈骗举报专区 | 涉历史虚无主义有害信息举报专区 | 涉企侵权举报专区
违法和不良信息举报电话:027-86699610 举报邮箱:58377363@163.com