
题目列表(包括答案和解析)
| 1 | 1 H 氢 1.008 |
2 He 氦 4.003 | ||||||
| 2 | 3 Li 锂 6.941 |
4 Be 铍 9.012 |
5 B 硼 10.81 |
6 C 碳 12.01 |
7 N 氮 14.01 |
8 O 氧 16.00 |
9 F 氟 19.00 |
10 Ne 氖 20.18 |
| 3 | 11 Na 钠 22.99 |
12 Mg 镁 24.31 |
13 Al 铝 26.98 |
14 Si 硅 28.09 |
15 P 磷 30.97 |
16 S 硫 32.06 |
17 Cl 氯 35.45 |
18 Ar 氩 39.95 |
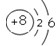
| 族/周期 | ⅠA | ⅡA | ⅢA | ⅣA | ⅤA | ⅥA | ⅦA | 0 |
| 第二 周期 |
3 Li 锂 6.941 |
4 Be 铍 9.012 |
5 B 硼 10.8l |
① | 7 N 氮 14.0l |
8 O 氧 16.00 |
9 F 氟 19.00 |
10 Ne 氖 20.18 |
| 第三 周期 |
11 Na 钠 22.99 |
② 24.31 |
13 Al 铝 26.98 |
14 Si 硅 28.09 |
③ 30.97 |
16 S 硫 32.06 |
17 Cl 氯 35.45 |
18 Ar 氩 39.95 |


| 族 周期 |
ⅠA | ⅡA | ⅢA | ⅣA | ⅤA | ⅥA | ⅦA | 0 |
| 第二 周期 |
3 Li 锂 7 |
4 Be 铍 9 |
5 B 硼 11 |
① | 7 N 氮 14 |
8 O 氧 16 |
9 F 氟 19 |
10 Ne 氖 20 |
| 第三 周期 |
11 Na 钠 23 |
② | 13 Al 铝 27 |
14 Si 硅 28 |
③ | 16 S 硫 32 |
17 Cl 氯 35.5 |
18 Ar 氩 40 |

(9分)我国制碱工业的先驱侯德榜将制碱与制氨结合起来的联合制碱法,为纯碱和氮肥工业技术的发展做出了杰出的贡献。其生产工艺流程示意图如下:
【资料】四种盐在不同温度下的溶解度表
| | 10℃ | 20℃ | 30℃ | 40℃ | 50℃ |
| NaCl | 35.8 | 36.0 | 36.3 | 36.6 | 37.0 |
| NH4HCO3 | 15.8 | 21.0 | 27.0 | —— | —— |
| NaHCO3 | 8.1 | 9.6 | 11.1 | 12.7 | —— |
| NH4Cl | 33.3 | 37.2 | 41.4 | 45.8 | 50.4 |
湖北省互联网违法和不良信息举报平台 | 网上有害信息举报专区 | 电信诈骗举报专区 | 涉历史虚无主义有害信息举报专区 | 涉企侵权举报专区
违法和不良信息举报电话:027-86699610 举报邮箱:58377363@163.com