
题目列表(包括答案和解析)
1g氢气燃烧生成液态水,放出142.9KJ热量,下列表示该反应的热化学方程式正确的是
[ ]
A.H2(g)+![]() O2(g)
O2(g)![]() H2O(l) △H=-285.8kJ/mol
H2O(l) △H=-285.8kJ/mol
B.2H2(g)+O2(g)![]() 2H2O(l) △H=-142.9kJ/mol
2H2O(l) △H=-142.9kJ/mol
C.2H2+O2![]() H2O △H=-571.6kJ/mol
H2O △H=-571.6kJ/mol
D.2H2(g)+O2(g)![]() 2H2O(l) △H=+571.6kJ/mol
2H2O(l) △H=+571.6kJ/mol
1g氢气燃烧生成液态水,放出142.9kJ热量,下列表示该反应的热化学方程式正确的是
[ ]
A.2H2(g)+O2(g)=2H2O(l);ΔH=-142.9kJ·mol-1
B.H2(g)+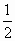 O2(g)=H2O(l);ΔH=-285.8kJ·mol-1
O2(g)=H2O(l);ΔH=-285.8kJ·mol-1
C.2H2(g)+O2=2H2O(l);ΔH=-571.6kJ·mol-1
D.2H2(g)+O2(g)=2H2O(l);ΔH=+571.6kJ·mol-1
1g H2燃烧生成液态水放出142.9kJ的热量,下列热化学方程式正确的是( )
A.2H2(g)+ O 2(g) =" " 2H2O(1)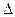 H =" " —142.9kJ·molˉl H =" " —142.9kJ·molˉl |
B.2H2(g)+ O 2(g) =" " 2H2O(1)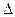 H =" " —571.6kJ·molˉl H =" " —571.6kJ·molˉl |
C.2H2+O2 = 2H2O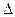 H = —571.6lkJ·molˉl H = —571.6lkJ·molˉl |
D.H2(g)+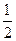 O 2(g) = H2O(1) O 2(g) = H2O(1)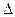 H = +285.8kJ·molˉl H = +285.8kJ·molˉl |
1g H2燃烧生成液态水放出142.9kJ的热量,下列热化学方程式正确的是
A.2H2(g)+ O 2(g) = 2H2O(1)
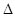 H = —142.9kJ·molˉl
H = —142.9kJ·molˉl
B.2H2(g)+ O 2(g) = 2H2O(1)
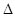 H = —571.6kJ·molˉl
H = —571.6kJ·molˉl
C.2H2+O2 = 2H2O
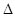 H = —571.6lkJ·molˉl
H = —571.6lkJ·molˉl
D.H2(g)+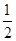 O 2(g) = H2O(1)
O 2(g) = H2O(1) 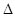 H = +285.8kJ·molˉl
H = +285.8kJ·molˉl
1g H2燃烧生成液态水放出142.9kJ的热量,下列热化学方程式正确的是( )
A.2H2(g)+
O 2(g) = 2H2O(1) 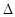 H =
—142.9kJ·molˉl
H =
—142.9kJ·molˉl
B.2H2(g)+ O 2(g) =
2H2O(1) 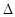 H =
—571.6kJ·molˉl
H =
—571.6kJ·molˉl
C.2H2+O2 = 2H2O
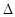 H =
—571.6lkJ·molˉl
H =
—571.6lkJ·molˉl
D.H2(g)+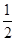 O
2(g) = H2O(1)
O
2(g) = H2O(1) 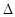 H =
+285.8kJ·molˉl
H =
+285.8kJ·molˉl
湖北省互联网违法和不良信息举报平台 | 网上有害信息举报专区 | 电信诈骗举报专区 | 涉历史虚无主义有害信息举报专区 | 涉企侵权举报专区
违法和不良信息举报电话:027-86699610 举报邮箱:58377363@163.com