
题目列表(包括答案和解析)
已知:C(s)+O2(g)====CO2(g) ΔH=-393.5 kJ·mol-1; CO(g)+1/2O2(g) ====CO2(g)
ΔH=-283 kJ·mol-1;则C(s)与O2(g)反应生成1 mol CO(g)的反应热为( )
A.ΔH=-676.5 kJ·mol-1 B.ΔH=+676.5 kJ·mol-1
C.ΔH=-110.5 kJ·mol-1 D.ΔH=+110.5 kJ·mol-1
已知:C(s)+O2(g)====CO2(g) ΔH=-393.5 kJ·mol-1; CO(g)+1/2O2(g) ====CO2(g)
ΔH=-283 kJ·mol-1;则C(s)与O2(g)反应生成1 mol CO(g)的反应热为( )
A.ΔH=-676.5 kJ·mol-1 B.ΔH=+676.5 kJ·mol-1
C.ΔH=-110.5 kJ·mol-1 D.ΔH=+110.5 kJ·mol-1
已知:C(s)+O2(g)====CO2(g)ΔH=-393.5 kJ·mol-1;CO(g)+1/2O2(g) ====CO2(g)
ΔH=-283 kJ·mol-1;则C(s)与O2(g)反应生成1 mol CO(g)的反应热为( )
A.ΔH=-676.5 kJ·mol-1 B.ΔH=+676.5 kJ·mol-1
C.ΔH=-110.5 kJ·mol-1 D.ΔH=+110.5 kJ·mol-1
已知:① C (s) + 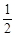 O2 (g)
O2 (g)
CO (g) △H = – 110.5 kJ ·
mol – 1
② C (s) + O2 (g) CO2
(g) △H = –
393.51 kJ · mol – 1
则反应:C (s) +
CO2 (g) 2CO (g) 的△H为(
)
A.– 283.01 kJ · mol – 1 B.+ 172.51 kJ · mol –1
C.+ 283.01 kJ · mol – 1 D.+ 504.00 kJ · mol – 1
湖北省互联网违法和不良信息举报平台 | 网上有害信息举报专区 | 电信诈骗举报专区 | 涉历史虚无主义有害信息举报专区 | 涉企侵权举报专区
违法和不良信息举报电话:027-86699610 举报邮箱:58377363@163.com