
32. I have worked with him for some time and have found that he is _______ than John.
A. more efficiently a worker B. a more efficient worker
C. more an efficient worker D. a worker more efficiently
31. There is no light in the dormitory. They must have gone to the lecture, _______?
A. didn't they B. don' t they C. mustn't they D. haven' t they
30. No one in the department but Tom and I __ that the director is going to resign.
A. knows B. know C. have known D. am to know
29.You might just as well tell the manufacturer that male customers _______ not like the design of the furniture.
A. must B. shall C. may D. need
28. As a rule, domestic servants doing odd jobs are paid _______.
A. by the hour B. by hour C. by an hour D. by hours
27. The village is far away from here indeed. It's ________ walk.
A. a four hour B. a four hour's C. a four-hours D. a four hours'
26. I am sorry it' s ______ my power to make a final decision on the project.
A. over B. above C. off D. beyond
25. Some of the stamps belong to me, while the rest are _________.
A. him and her B. his and hers C. his and her D. him and hers
Part A Short Conversations
1.A. At 12:00. B. At 12:15. C. At 12:30. D. At 12:45.
2. A. To visit a museum. B. To attend a wedding. C. To get married. D. To go to India.
3. A. Developing a film. B. Watching a movie. C. Repairing a camera. D. Taking a photo.
4. A. In a tea house. B. In a school. C. In a grocery. D. In a garage.
5. A. The desk lamp. B. The electricity bill. C. The dirty kitchen. D. The power failure.
6. A. She could have done better in the interview.
B. She couldn't answer some of the questions.
C. She was quite pleased with the interview.
D. She was disappointed with the interview.
7. A. A couple. B. Neighbours. C. Classmates. D. Colleagues.
8. A. She saw the play more than once. B. She acted in the play.
C. She visited the English Department. D. She led the drama club.
9. A. Minor changes could be made. B. Major corrections are needed.
C. The paper should be rewritten. D. The paper needs no revision.
10. A. Engineering. B. Education. C. Manufacturing. D. Business.
Part B Passages
Questions 11 through 13 are based on the following passage.
11. A. He was robbed. B. He was knocked down by a bus.
C. He fell ill suddenly. D. He was chased by some tough guys.
12. A. A neighbour. B. A stranger. C. A friend. D. A doctor.
13. A. A friend in need is a friend indeed.
B. Neighbours are clearer than distant relatives.
C. Church-goers are very helpful.
D. Only doctors can save our lives.
Questions 14 through 16 are based on the following news.
14. A. Britain. B. France. C. Sweden. D. Spain.
15. A. J. K. Rowling will have an Internet interview.
B. Children will meet Harry Potter's mother.
C. The book will be available on the Internet.
D. The book will arrive in China in early June.
16. A. To get ready for a military parade. B. To learn to protect themselves.
C. To gain some military knowledge. D. To develop their love for the country.
Part C Longer Conversations
Blanks 17 through 20 are based on the following conversation.
|
Flight
Reservation Form |
|
Departure time:
July 23rd Time of return: July 17 Place of arrival: 18 Price: $ 980 Flight number: 19 Requirement: A seat 20 |
Complete the form. Write NO MORE THAN THREE WORDS for each answer.
Blanks 21 through 24 are based on the following conversation.
|
Where will the new students meet at 10
am? |
In the 21 . |
|
What will the Director of Studies tell
them about? |
About the 22 and their requirements. |
|
Who will give a talk about the services
and activities? |
The 23
. |
|
What will they do in Classroom 5? |
To have an 24 . |
Complete the form. Write NO MORE THAN TWO WORDS for each answer.
29.(14分)钒(V)及其化合物广泛应用于工业催化、新材料和新能源等领域.
(1)V2O5是接触法制硫酸的催化剂.
①一定条件下,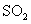 与空气反映t min后,
与空气反映t min后,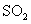 和
和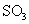 物质的量浓度分别为a mol/L和b mol/L, 则
物质的量浓度分别为a mol/L和b mol/L, 则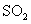 起始物质的量浓度为
mol/L ;生成
起始物质的量浓度为
mol/L ;生成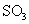 的化学反应速率为
mol/(L·min) .
的化学反应速率为
mol/(L·min) .
②工业制硫酸,尾气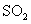 用_______吸收.
用_______吸收.
(2)全钒液流储能电池是利用不同价态离子对的氧化还原反应来实现化学能和电能相互转化的装置,其原理如题29图所示.

①当左槽溶液逐渐由黄变蓝,其电极反应式为 .
②充电过程中,右槽溶液颜色逐渐由 色变为 色.
③放电过程中氢离子的作用是 和 ;充电时若转移的电子数为3.01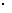 1023个,左槽溶液中n(H+)的变化量为 .
1023个,左槽溶液中n(H+)的变化量为 .
29.答案(14分)
(1)① ;
;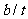
②氨水
(2)①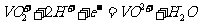
②绿 紫
③参与正极反应; 通过交换膜定向移动使电流通过溶液;0.5mol
[解析]本题考查以钒为材料的化学原理题,涉及化学反应速率和电化学知识。
(1)
由S守恒可得,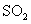 的起始浓度为(a+b)mol/L。
的起始浓度为(a+b)mol/L。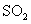 的速率为单位时间内
的速率为单位时间内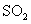 浓度的变化,即b/tmol/(L﹒min)。
浓度的变化,即b/tmol/(L﹒min)。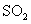 可以用碱性的氨水吸收。
可以用碱性的氨水吸收。
(2)
①左槽中,黄变蓝即为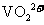 生成
生成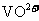 ,V的化合价从+5降低为+4,得一个电子,0原子减少,从图中知,其中
,V的化合价从+5降低为+4,得一个电子,0原子减少,从图中知,其中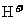 发生了移动,参与反应,由此写成电极反应式。②作为原电池,左槽得电子,而右槽失电子。充电作为电解池处理,有槽中则为得电子,对应化合价降低,即为
发生了移动,参与反应,由此写成电极反应式。②作为原电池,左槽得电子,而右槽失电子。充电作为电解池处理,有槽中则为得电子,对应化合价降低,即为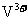 生成
生成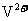 ,颜色由绿生成紫。③由电极反应式知,
,颜色由绿生成紫。③由电极反应式知,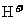 参与了反应。溶液中离子的定向移动可形成电流。n=N/NA=3.01×
参与了反应。溶液中离子的定向移动可形成电流。n=N/NA=3.01×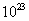 /6.02×
/6.02×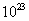 =0.5mol。
=0.5mol。
[规律总结]电化学试题的分析一般是从化合价着手,对于原电池,化合价升高的作为负极,化合价降低的作为正极,两极方程式相加即可得总反应。对于电解池,化合价升高作为阳极,降低的作为阴极。两者之间的关系是:正极反应式颠倒即为阳极反应式,负极反应式颠倒即为阴极反应式。
湖北省互联网违法和不良信息举报平台 | 网上有害信息举报专区 | 电信诈骗举报专区 | 涉历史虚无主义有害信息举报专区 | 涉企侵权举报专区
违法和不良信息举报电话:027-86699610 举报邮箱:58377363@163.com