��2013?�㶫ģ�⣩��ѧ��һ����ʵ��Ϊ�����Ŀ�ѧ����ѧ��ȡ�õķ�˶�ɹ�������ʵ�����Ҫ���÷ֲ����ģ��������ʵ��װ��ͼ1�ش����⣺
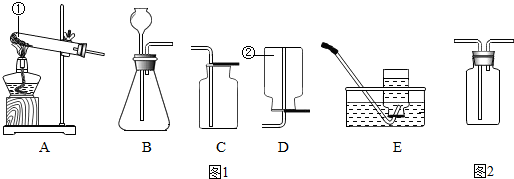
��1��д��ָ�����������ƣ���
�Թ�
�Թ�
����
����ƿ
����ƿ
��
��2��ʵ��������ˮ�����ƹ��塢NaOH�ͼ�ʯ�ҹ�����ȡ�ܶȱȿ���С��������ˮ�ļ��飬��ѡ�õķ���װ����
A
A
��ѡ����ĸ����ͬ������ѡ�õ��ռ�װ����
D��E
D��E
��
��3��ʵ���ҿ���B��Eװ����ȡ��������
����������
����������
���÷�Ӧ�Ļ�ѧ����ʽΪ
Zn+H
2SO
4=ZnSO
4+H
2����2H
2O
22H
2O+O
2��
Zn+H
2SO
4=ZnSO
4+H
2����2H
2O
22H
2O+O
2��
��
��4��ʵ����ͨ������ͼ2��ʾ��ϴ��װ�ö��������и��ϴ��ƿ������װ��ҩƷ������
A
A
��ѡ����ĸ����
A��Ũ���� B������������Һ C����ʯ�� D���Ȼ��ƹ��壮





 ��2013?�㶫ģ�⣩��
��2013?�㶫ģ�⣩�� ������
������ ������
������ ���ֱ��ʾA��B��C�������ʵ�ԭ�ӣ����й�����ͼ˵����ȷ���ǣ�������
���ֱ��ʾA��B��C�������ʵ�ԭ�ӣ����й�����ͼ˵����ȷ���ǣ�������