ij��ѧ��ȤС����Ƴ����д����ᴿ��ʵ�鷽��������һ��������������⣮
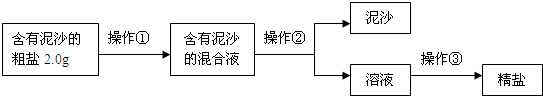
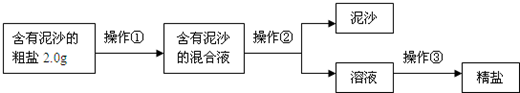
��1�������٢ڢ۵�����������
����
����
��
����
����
��
����
����
����ʵʩ������ǰ���Ƚ�����ĥ�飬��������
�����ܽⲢ��ȥ���е�����
�����ܽⲢ��ȥ���е�����
��ͬʱ�ò���������ˮ���������
B
B
����֪20��ʱʳ�ε��ܽ����36g������ĸ��ţ���
A 5mL B 8mL C 12mL
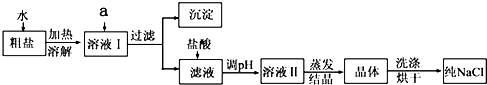
��2������������IJ���������
�ձ���©����������
�ձ���©����������
�����ò�������������Һ�Ի��ǣ������ԭ����
��ֽ����
��ֽ����
���δ�һ��ԭ���ɣ���
��3��ʵʩ������ʱ������Ҫ����
����
����
���������������ֱ��
�ֹ���ᾧ����
�ֹ���ᾧ����
ʱֹͣ���ȣ�
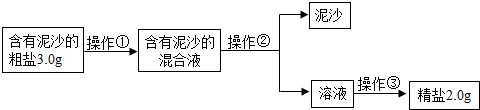
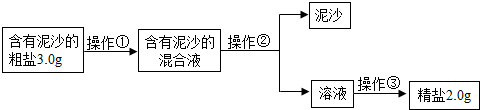
��4����ȤС���ͬѧͨ����ȷ�ļ��㷢�֣�ʵ�����þ��εIJ��ʱȸô������Ȼ��Ƶ�ʵ�ʺ���ƫ�ͣ�����ܵ�ԭ����
BCE
BCE
������ĸ��ţ�
A������ʱ��ֽ������ B������ʱ�й��彦��
C���ܽ⺬����ɳ�Ĵ���ʱ�������ˮ������
D����������ɳ�Ļ��Һȫ���㵹�������������ˮϴ�Ӳ��������ձ�������Һ��һ�����������
E��ת�ƾ���ʱ�������������������в����Ĺ���
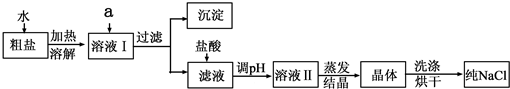
��5����ȤС���Ա�������Ϻ��֪�������г���NaCl����ɳ�⣬����������MgCl
2��CaCl
2��Na
2SO
4�����ʣ���������һ�ֽ�
��ɳ
��ɳ
��ˮ����ķ����������һ��ʵ�鷽�����ܳ�ȥ�����е�MgCl
2��CaCl
2��Na
2SO
4�����ʣ�Ϊ�ˣ���������������˵ڶ���ʵ�鷽����
�ṩ���Լ��У�Na
2CO
3��Һ��K
2CO
3��Һ��NaOH��Һ��BaCl
2��Һ��Ba��NO
3��
2��Һ������NaCl��Һ��
������ȥ��ҺI�е�MgCl
2��CaCl
2��Na
2SO
4�����ṩ���Լ���ѡ��a���������Լ������μ�˳������Ϊ��������NaOH��Һ��������
BaCl2��Һ
BaCl2��Һ
��������
Na2CO3��Һ
Na2CO3��Һ
��
������Һ�м������������
��ȥ������̼���ƺ���������
��ȥ������̼���ƺ���������
��
��6������ȤС���������ᴿ��ľ�������200g������������Ϊ0.9%��������ˮ����Ҫ���ε�����Ϊ
1.8
1.8
g�����Ƹ���Һʱ����Ҫ��ˮӦѡ��
500mL
500mL
��ѡ�50mL������100mL����500mL������Ͳ������ʱ�����������ӣ��������������������Ƶ���Һ��������������
��
��
��ѡ����ڡ�����С�ڡ����ڡ���0.9%��