
��ͼ�Ǽס��ҡ����������ʵ��ܽ�����ߣ��ش��������⣺

��1��P���ʾ��������__________________________��
��2��t3��ʱ��50g�������ܽ���50gˮ�У��õ���Һ��������____________��
��3���ס��ҡ����������ʵı�����Һ��t2�潵�͵�t1��ʱ���õ����������ʵ����������Ӵ�С��˳����______________��
��4��Ҫʹ�����ʵı�����Һ��Ϊ��������Һ���ڲ��ı����ʵ���������£�Ӧ��ȡ�ķ�����______________��
��5������˵����ȷ���� ��_____��
A.P���DZ�ʾ��t2��ʱ���ס��ҵ������������
B.t3��ʱ����100gˮ������ܽ�50g�ļ�����
C.t1��ʱ����50gˮ�м���15ga���ʣ��õ���Һ65g
D.���ܽ�ȴ��ڱ����ܽ��

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ��2017��㶫ʡ��������������У���꼶�п�һģ��ѧ�Ծ��������棩 ���ͣ�ѡ�������
��ѧ�����ཡ����ϵ���У�����˵����ȷ����
A. �ӻ��������ĽǶȿ��ǣ������ȼ����ú
B. Ϊ��Ԥ��ƶѪ������㡢����ʳ�ﲹ��������Ҫ�ĸ�Ԫ��
C. �߲ˡ�ˮ����ʳ���������ȡά���ص���Ҫʳ��
D. �ü�ȩ��Һ�������ʣ��ӳ�ʳ�ﱣ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2017�����ʡ֦���о�У���꼶��ѧ������������ѧ�Ծ��������棩 ���ͣ�ѡ�������
����������������ǿ���ǣ� ��

A. ƻ��֭ B. ţ�� C. ����ˮ D. ¯������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2017�����ɹŶ�����˹�б�ҵģ�⿼�Ի�ѧ�Ծ��������棩 ���ͣ�ѡ�������
����ʵ�鷽���У���������
A. �Ƚ�п�����Ļ�ԣ���пƬ����Ƭ�ֱ��������ͭ��Һ��
B. �����������ƺ��������ƿ���壺��ƿ�ڼ�������ˮ�����¶ȱ仯
C. ��ȥϡ�����е���������������������ᱵ��Һ����ַ�Ӧ�����
D. ����Cu(OH)2��NaNO3�Ĺ������ȡ��������������ˮ�ܽ⣬����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2017�콭��ʡ���꼶�п�Ѻ�⻯ѧ�Ծ��������棩 ���ͣ�̽����
̼�����ƣ�NaHCO3������С�մ�����ʳƷ��ҽѧ��ҵ��ij��ѧ��ȤС���ͬѧ��̼�����Ƽ��Ⱥ�IJ���ܸ���Ȥ���Բ�����з�������ͼ����

��1�����Կ���ʵ�������У��Թܿ���Сˮ���γɣ������ʯ��ˮ����ǣ��ó���������
_________��__________��
��2����ȤС����̽����ɫ��������
��������⡿���ɫ������ʲô��
���������ϡ��Ȼ�����Һ������
��������롿
����1����ɫ������̼����
����2����ɫ��������������
����3����ɫ������_____________________
��ʵ��̽����
��ȤС��Ϊ��ȷ����Ӧ��Ĺ������ɷ֣�����������ʵ�飬����д�±���
ʵ�鷽�� | ʵ������ | ���� |
����һ��ȡ������Ӧ��Ĺ����������ˮ������������Ȼ�����Һ������ | ������ɫ���� | ����1���� |
�������ȡ��Һ����������Һ�е��� ____________________________ | __________________ |
��ʵ���ܽд��̼���������ȷֽ�Ļ�ѧ����ʽ��__________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2017�콭��ʡ���꼶�п�Ѻ�⻯ѧ�Ծ��������棩 ���ͣ�ѡ�������
����ʵ������������ȷ���ǣ�_____��
A.��ʢ��Ũ������Լ�ƿ��ƿ�ǣ�ƿ�ڳ��ְ���
B.һ����̼��ԭ����ͭ�����ɺ�ɫ����
C.���ڿ����о���ȼ�ա��������䣬�ų��������ȣ����ɺ�ɫ����
D.��������ϡ���ᷴӦ����Һ����ɫ���_______________
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2017�콭��ʡ���꼶�п�Ѻ�⻯ѧ�Ծ��������棩 ���ͣ�ѡ�������
�й���Һ���Ʋ�������˳����ȷ���ǣ� ��
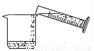

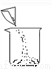
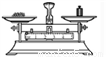

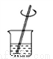
�� �� �� �� �� ��
A. �٢ڢۢܢݢ� B. �ݢܢڢ٢ۢ�
C. �ڢ٢ݢܢۢ� D. �ݢܢۢڢ٢�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2017�콭��ʡ�����о��꼶��ѧ�����п��Ի�ѧ�Ծ��������棩 ���ͣ�ѡ����
���и���ϡ��Һ�У����������Լ����ܼ����������
A. Na2CO3��H2SO4��HCl��NaNO3 B. HCl��K2CO3��BaCl2��Na2SO4
C. CuSO4��NaOH��KNO3 ��KOH D. NaOH��NaCl��HCl��FeCl3
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2017��㶫ʡ����У���꼶��ѧ������������ѧ�Ծ��������棩 ���ͣ������
ij��ʴӡˢ��·��ķ�Һ�к���CuCl2��FeCl2��Ϊ�˷����÷�Һ����ɣ�ȡ1 000 g��Һ���������ʵ�鷽������̽����

ʵ������м������м������������Cu�����������ݼ�¼���±���
���������/g | 20 | 40 | 60 | 80 |
����Cu������/g | 12.8 | 25.6 | 32 | 32 |
��ע������м�е����ʲ����ڷ�ҺҲ�����Һ��Ӧ�������ǹ����е���ʧ����
��1��1 000 g��Һ����������м��ȫ��Ӧ������Cu������Ϊ_______ g��
��2������м�е���Fe����������Ϊ_______________��
��3������1 000 g��Һ��CuCl2����������_______����д��������̣��������С�����һλ��
��4��1 000 g��Һ��FeCl2����������Ϊ_________�����������С�����һλ��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com