
ijУ��ѧ����С����һ�λ�У�ȡ�ս����������ˮˮ����ÿ��5������pH�Ʋⶨһ��pH�����������±���
�ⶨʱ�� 6��05 6��10 6��15 6��20 6��25 6��30
p H 4.95 4.94 4.94 4.88 4.86 4.85
��֪��������ˮ��pHԼΪ5.6�������pHС��5.6��
��1����ȡ����ˮ�Ƿ�Ϊ���ꣿ��������ǡ������ڲⶨ���ڼ䣬��ˮˮ������������ʱ��ı仯�����������ǿ������������
��2�������飬��һ������һ��ȼú���糧�����������в���SO2����ijͬѧ����û�����������������в�����SO2������ת�����������ƣ�Na2SO3�����������д���÷�Ӧ�Ļ�ѧ����ʽ��������
��3������pH��ֽ�ⶨ��ˮ�����ȣ��ⶨ�����ǣ���������
�����㡿����IJ�����Σ�������Σ���Һ���������pHֵ�Ĺ�ϵ����Һ������Բⶨ����д��ѧ����ʽ�����ֱ���ʽ�����뷽��ʽ��
��ר�⡿��Ͽα�֪ʶ����Ϣ��ѹ��ʵ���⣮
��������pH��5.6����ˮ��Ϊ���ꣻ����Һ��pH��7ʱ������pH�ļ�С������ǿ�����ݷ�Ӧ��������P�������غ㶨�ɿ�����д��ѧ����ʽ����pH��ֽ���Բⶨ��Һ�����ȣ�
����𡿽⣺��1����ˮ��pHС��5.6���������꣬�ڲⶨ���ڼ䣬��ˮˮ����pH��С��������ǿ������ǣ���ǿ��
��2������������������Ʒ�Ӧ�����������ƺ�ˮ����Ӧ�Ļ�ѧ����ʽΪ��SO2+2NaOH�TNa2SO3+H2O��
��3����pH��ֽ�ⶨ��ˮ�����ȵķ����ǣ��ò�����մȡ��ˮ��Ʒ����pH��ֽ�ϣ���pH��ֽ����ʾ����ɫ�����ɫ���Աȣ�������ֵ��
�������������Ҫ���ջ�ѧ����ʽ����д�����Ͳⶨ��Һ���ȵķ����ȷ����֪ʶ��ֻ���������ܶ���ط��������������ǿ���жϣ�
��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���в����ڻ��Ϸ�Ӧ��Ҳ�����ڷֽⷴӦ��������������Ӧ���ǣ�������
A��ľ̿+����
 ������̼
������̼
B����+����
 ������
������
C��ʯ��+����
 ˮ+������̼
ˮ+������̼
D��������
 ��+����
��+����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��֪A��B�ɷ����кͷ�Ӧ���ڳ�����DΪҺ�壬X��ĿǰӦ����㷺�Ľ�����Y�� ����ʳƷ������������ʼ��ת����ϵ��ͼ��ʾ��
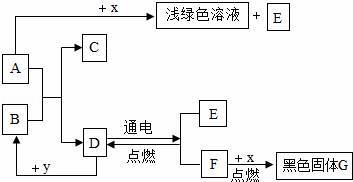

��1��E�Ļ�ѧʽΪ�������о�E����;һ����������
��2��D��Y��Ӧ�Ļ�ѧ����ʽΪ����
��3��D��Ӧ����E��F�Ļ�ѧ����ʽΪ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���и������ʣ�ֻ����ɫ��̪��Һ�����Ӧ�������������ǣ�������
A��NaOH��HCl��H2SO4 B��NaOH��HCl��NaCl
C��Na2CO3��HCl��NaCl D��NaOH��Ca��OH��2��Ba��OH��2
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�����ĸ�ͼ��ֱ��Ӧ�ĸ��仯���̣�������ȷ���ǣ�������
A��
 һ�����ı���ʯ��ˮ�м���������
һ�����ı���ʯ��ˮ�м���������
B��

�����£���ͬ������п�����ֱ�����������������������ͬ��ϡ���ᷴӦ
C��

���������Ũ�ȵ�˫��ˮ��ȡ����
D��

����θ��ƽ[��Ҫ�ɷ�Al��OH��3]����θ����࣬θҺpH�ı仯
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
Ϊ�ⶨijʯ��ʯ��Ʒ��̼��Ƶĺ�����С������ʵ�飬��������������ͼ��ʾ��


��1����ȫ��Ӧ���ɶ�����̼������Ϊ������
��2����ʯ��ʯ��Ʒ��̼��Ƶ����������Ƕ��٣�
��3����Ӧ���������������Ƕ��٣�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��AgNO3��Cu( NO3)2�����Һ�м���һ������п�ۣ���ַ�Ӧ���ˣ��������м���ϡ������������������˵����ȷ���ǣ� ��
A.һ����п��ʣ�� ��������������������B.������Һ��һ����Zn2+��Cu2+
C.һ������������ D.������Һ��һ����Cu2+��Ag+
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�����£�ij����������ˮ���ܶȱȿ������ռ�������ɲ��õķ����ǣ�������
A�������ſ����� B����ˮ������
C����ˮ�������������ſ����� D����ˮ�������������ſ�����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2016�콭��ʡ̩���о��꼶��ѧ����ĩ���Ի�ѧ�Ծ��������棩 ���ͣ������
������ճ������ũҵ�����벻��ˮ����ش�
��1�� ���н϶�����Ըơ�þ�������ˮ���� (���ˮ����Ӳˮ��)��
��2�� ���о���ˮ�ĵ�һ�����У���Ծ����̶Ƚϸߵ��� (����ĸ)��
A������ B���������� C. ����
��3�� ˮ��ͨ��������¿��Էֽ⣬д���÷�Ӧ�Ļ�ѧ����ʽ ��
��4�� �������ֹ������ʵ��ܽ����������ͼ��ʾ���ش��������⣺

���� ��ʱ�������������ʵ��ܽ����ȣ�
�ڽ�t1��ʱ�������ļ����������ʵı�����Һ�ֱ��µ�0�棬���������������ٵ��� (��ס����ҡ�)��
��t2��ʱ����60 g�����ʷ���100 g ˮ�У���ֽ��裬������Һ��������������w(��)��ͬ���������ʵı�����Һ��������������w(��)��С��ϵΪ (����ĸ)
A.w(��)< w(��) B.w(��)> w(��)
C.w(��)= w (��) D.��ȷ��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com