
(13��)ij��ȤС����������װ�ý��У�����CO�� ��ʵ�����Ʒ����й����ʵ��о���
(1)д�����б�����������ƣ���________��________��
(2)��ͬѧҪ�ø����������ȡ������ Ӧѡ����ͼ�е�_________װ��(����)���÷�Ӧ�Ļ�ѧ����ʽ�ǣ�____________���������ռ�����ȡ������ƿ��ֹͣ��ʵ�����ȷ�������Ⱥ�˳��Ϊ_____________��
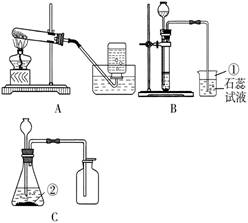
(3)��������֪��Ũ������лӷ��ԣ��ܻӷ����Ȼ��⣮
����ͬѧ��Ũ����ʹ���ʯ�ڣ�װ���з�Ӧ����ȡCO�����������й�����ʱ�۲쵽�ձ�����ɫʯ����Һ��죮����һ�仯�ĺ���������_____(�����)��
A��������CO��ֱ��ʹʯ����Һ���
B��������CO����ˮ��Ӧ����H��CO��ʹʯ����Һ���
C���ӷ������Ȼ�������ˮʹʯ����Һ���
����ͬѧ���Ƶõ�����ͨ������ʯ��ˮ�У�ʯ��ˮû�б���ǣ���ԭ����____________��
��4����ͬѧ�ã�װ����ȡ��һƿ��ɫ����ζ������(������)�����Ʋ�����������___________����������ķ���֤ʵ����Ʋ�_________________________________��
��5����ͬѧ����ͼ��ʾװ�����һϵ�е�ʵ�飮��ʵ��װ�õ���Żش���������(β������װ��δ����)�����ϣ���ɫ������ͭ��ĩ��ˮ�����ɫ��
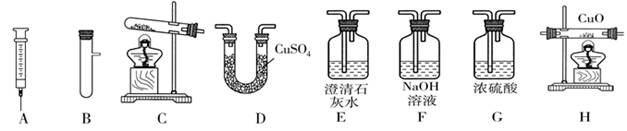
��ʵ����������A��B����ϳ���ȡ�����ķ�Ӧװ�ã�������Ӧ�Ļ�ѧ����ʽΪ_______��
�ڼ���CO�л��е�CO����װ����_______����ȥCO�н϶��CO�������ѡװ��______��
��ˮú������CO��������CO����H��O��֤��ˮú���к���CO�ͣ�����ʵ��װ�õ�����˳���ǡ�
( )��( )��( )��( )��( )��β������(��D��H��ѡ��)��
��1���ձ�����ƿ��2��A 2KMnO4 K2MnO4+MnO2+O2��
K2MnO4+MnO2+O2��
���Ƴ�ˮ�еĵ��ܣ���ֹͣ���ȣ�2����BC�ڶ�����̼�л����Ȼ�������
��3��������̼��ȡ������ʯ��ˮ��ʯ��ˮ�����
��4����2H2O2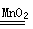 2H2O+O2����E F
2H2O+O2����E F
�ۡ�( F )��( G )��( H )��( D )��( E )��β������
����������2������װ��A�ռ���������ʱ��һ��Ҫ���Ƴ�ˮ�еĵ��ܣ���ֹͣ���ȣ���ֹˮ����ʹ�Թ�ը�ѣ���3��������̼����ʹʯ����Һ��ɫ��������̼����ˮ���ɵ�̼����ʹʹʯ����Һ��ɫ���Ȼ�����������ˮ������Ҳ��ʹʯ���ɫ������BC����������̼�л����Ȼ������壬���Ȼ��������������Ʒ�Ӧ����ʹʯ��ˮ������ǣ���4����Cװ�ù�Һ�����ͣ��������ſ������ռ����壬���Ƕ�����̼��������̼�ļ��鷽����ȡ������ʯ��ˮ��ʯ��ˮ����ǣ���5��Ҫ֤��ˮú���к���һ����̼�ͣ�����Ӧ��ȥ������̼��ˮ�֣���ͨ�����ȵĽ��������ͨ�����������������һ����̼�������Ĵ���



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
| 1����̼���ƾ���̼���ƺ��������˫�����ʣ����ȶ������ȡ���ˮ�ֽ⣻ 2����̼���Ʒֽ�Ļ�ѧ����ʽΪ2��2 Na2CO3?3 H2O2 ��=4 Na2CO3+6 H2O+3O2�� 3��H2O2�ֽ�ų�������Na2CO3���Ȳ��ֽ⣻���Ʊ��о�����ͼ1���Ʊ���̼���ƵĹ������̣�  ��ش����⣺ ��1����Ӧ���з�����Ӧ�Ļ�ѧ����ʽΪ 2Na2CO3+3H2O2�T2Na2CO3?3H2O2 2Na2CO3+3H2O2�T2Na2CO3?3H2O2 ����2�������У���Ӧ����������ڽϵ��¶��½��У�ԭ���� ��ֹ��̼���Ʒֽ⣬Ӱ������Ͳ��� ��ֹ��̼���Ʒֽ⣬Ӱ������Ͳ��� ����3�����������У������϶��NaCl���Ա��̼���ƾ���������������Ϊ ��̼������NaCl��Һ���ܽ�Ƚ�С������������ ��̼������NaCl��Һ���ܽ�Ƚ�С������������ ����4������A�������� ���� ���� ������ʵ������ɴ˲�������Ҫ�IJ����������ձ��������� ������ ��©�� ©�� ����5��ϴ�ӹ�̼���ƾ������ѡ�� B B ��A������ˮ B����ˮ�ƾ� C������̼������Һ D������NaCl��Һ ��6��ĸҺ����ѭ��ʹ�ã�������Ҫ���е������� NaCl NaCl ��Na2CO3����H2O2�� Na2CO3����H2O2�� �����Ȳⶨ���ⶨ��̼������Ʒ�����ʲ�����ˮ����2Na2CO3?3H2O2������������ С��ͬѧ�������ͼ2��ʾʵ��װ�ã�����������ȥ����������ʵ�飮 [ʵ�鲽��]���� ��������� ��������� ���ڽ���̼������Ʒ��2.20g����������ɫ��ĩ���Ȼ�Ϻ�����װ��A�У�����Ͳ�м���ˮ����������������Һ©����������������ˮ���ܴ�װ��A�����ٲ������� ���ٲ������� ʱ��ֹͣ�μ�ˮ�����رջ������ݴ���Ͳ��ˮ�治�ٱ仯ʱ����¼����ˮ��̶ȣ��������ݽ��м��㣮[ʵ����������ݴ���]�� ��7������ʵ�鲽���������ݣ��� ��������� ��������� �������ٲ������� ���ٲ������� ����8��ʵ���У�ˮ�������� �ܽ��̼���ƣ�������ֽ� �ܽ��̼���ƣ�������ֽ� ����ɫ��ĩ�ܼӿ��̼���Ƶķֽ����ʣ������������ͻ�ѧ�����ڷ�Ӧǰ������䣬������MnO2����CuO�� MnO2����CuO�� ���ѧʽ������9��ʵ���У� A A ����ʼ�ռ����壻A���տ�ʼ��������ʱ B�������ݾ�����������ʱ C�����ô�����ľ�����ڵ��ܿڲ�����ȼʱ ��10������Ͳ���ռ�������������224mL��ͨ������£��������ܶȽ���Ϊ1.43g?L-1�����������̼������Ʒ��2Na2CO3?3H2O2����������[Mr��2Na2CO3?3H2O2��=314��Mr��O2��=32] 95% 95% ��[ʵ�鷴˼]�� ��11���������ۣ�С��ͬѧһ����Ϊ�ⶨ���ƫ����ԭ���� �����ˮռ������ƿ�ڵ������ʹ��O2���ƫ�� �����ˮռ������ƿ�ڵ������ʹ��O2���ƫ�� ����12����Ͳ�г����ܵ������� �����ã���ֹ�¶Ƚ���������ʱ�������������ƫ���������� �����ã���ֹ�¶Ƚ���������ʱ�������������ƫ���������� ����13����ͬѧ�����װ����ԭ�п�����ʹ�ⶨ���ƫ������˵���Ƿ���ȷ���粻��ȷ����˵�����ɣ� ����ȷ��װ����ԭ�п��������ɵ�O2��������� ����ȷ��װ����ԭ�п��������ɵ�O2��������� ����14���������ۣ�С��ͬѧһ����Ϊ���������������вⶨ����������װ��A��ʣ������ˣ�ϴ������������ϴ��Һ������Һ��������ˮ����1.39g������Ʒ��2Na2CO3?3H2O2����������Ϊ 93.6% 93.6% ����15���������Ͽ�Ƭ���㻹����Ƴ�ʲô�����ⶨ��Ʒ��2Na2CO3?3H2O2������������ �뷽�������̼���ƺ��ᷴӦ����CO2��������������������м��㣨���̼���ƺ��Ȼ��ơ��Ȼ�������Һ���ɳ����������вⶨ �뷽�������̼���ƺ��ᷴӦ����CO2��������������������м��㣨���̼���ƺ��Ȼ��ơ��Ȼ�������Һ���ɳ����������вⶨ ����ֻ�������Ҫ�ķ�����
�鿴�𰸺ͽ���>> ��Ŀ�����л�ѧ ��Դ��2011-2012��㶫ʡ�����з�����ѧ���꼶��ѧ���¿���ѧ�Ծ����������� ���ͣ�̽���� ��13�֣�ij��ȤС�����ʵ�����ṩ��������ҩƷ�������������Ʊ�ʵ�顣����������мг���������ʡ�ԣ��������ͼ�ش����⣺ �鿴�𰸺ͽ���>> ��Ŀ�����л�ѧ ��Դ��2012�콭��ʡ����������ѧ���꼶5���п�ģ�⻯ѧ�Ծ����������� ���ͣ�̽���� ��9�֣�ij��ȤС��ͬѧΪ֤��NaOH��Һ��ϡ���ᷢ�����кͷ�Ӧ���Ӳ�ͬ�Ƕ����������ʵ�鷽����������ʵ�顣
��ijͬѧ��ûʹ���¶ȼƵ�����£�ͨ����ͼ��ʾװ�������ʵ�顣���ͬѧ����ʵ������ ���ж�NaOH��Һ��ϡ���ᷢ�����кͷ�Ӧ�� 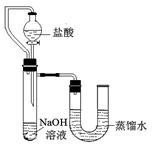 II��ʵ���е�������� ��ʵ������У����Ƿ���ʢ��NaOH��Һ���Լ�ƿƿ�ں���Ƥ���ϳ����˰�ɫ��ĩ������������ѧ�Ļ�ѧ֪ʶ�������ְ�ɫ��ĩ�ijɷ��������²��룺�ٿ�����NaOH���ڿ�����Na2CO3���ۿ�����NaOH��Na2CO3�� ��С��ͬѧȡ��ɫ��ĩ����������ˮ��������Һ�м��������� ��Һ��������ɫ����������Ȼ�����ϲ���Һ�м����̪��Һ��������Һ�ʺ�ɫ����֤�˲��������ȷ�ġ� ��Ϊ�˽�һ���о�����λͬѧȡ��10.0g������Ʒ�����õ�����ƽ��ͬ������ͼ��ʾ��ʵ�顣  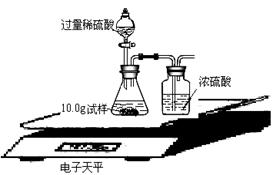 ʵ�����ݼ�¼���£�
��8����ͬѧ���������ʵ�������������Na2CO3����������ƫС����ͬѧ�������ǣ�ʵ���������ȷ���� �� �鿴�𰸺ͽ���>> ��Ŀ�����л�ѧ ��Դ��2011-2012��㶫ʡ�����о��꼶��ѧ���¿���ѧ�Ծ��������棩 ���ͣ�̽���� ��13�֣�ij��ȤС�����ʵ�����ṩ��������ҩƷ�������������Ʊ�ʵ�顣����������мг���������ʡ�ԣ��������ͼ�ش����⣺
��1��ͼ�������ܢݵ����ƣ��� ���� �� ��2��ʵ������ȡ������ijͬѧ�����ͼA��װ�ã������������ռ����������У���������ʢ�ŵ��Լ�ӦΪ ���˷����������Ļ�ѧ����ʽΪ ���ռ������ķ���Ϊ �����������ռ�����ʱ���������ڲⶨ����������� ��ʱ����Ҫ �����������ƣ��� ��3��ʵ������ȡ������̼����B~G��ѡ����������װ����װ�ã�Ҫ���ܷ�����Ʒ�Ӧ�ķ�����ֹͣ�������ȷѡ��Ϊ������ĸ�� �� ��4��ʵ��ǰ����ͬѧ�����������м���ˮ��Ȼ���ܷ���ˮ���У����������ٵĻ������۲쵼�ܿ��Ƿ������������ݳ��֡���ʵ�������Ŀ����__________�� ��5����ͬѧ��ȡһ������KMnO4���ڴ��Թ��У����¶ȿ�����250�������ȡO2 ��ʵ�����ʱ����ͬѧ�����ռ�����O2�������۲����������һ����ͬѧ�ǽ���������̽���� ��������롿����l����Ӧ���ɵ�MnO2�ֽ�ų�O2�� �����Ӧ���ɵ�K2MnO4�ֽ�ų�O2 �������������������_______________________________�� ��ʵ����֤��ͬѧ�Ƿ�Ϊ���飬�ֱ��������ʵ�飺 �ٵ�һ��ͬѧȡһ��������MnO2����250�������¼���һ��ʱ�䣬��ȴ����MnO2���������䡣�����________________���� �ڵڶ���ͬѧȡK2MnO4��250�������¼��ȣ�û���òⶨ�����ķ����ó��˲������ȷ�Ľ��ۡ�����ͬѧѡ���ʵ�鷽����_________________ ��
�鿴�𰸺ͽ���>> ͬ����ϰ��� ����ѧУ��ѡ - ��ϰ���б� - �����б� ����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר�� Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com��Ȩ��������վ�������£�ͼƬ��Դ�����磬����Ȩ����Ȩ��ԭ�������У�ת�������ַ���Ȩ��������Ȩ����������������֪�����ǽ����촦������ϵqq��3310059649�� ICP�������: ��ICP��07509807��-10 ����������42018502000812�� |