
ЎѕМвДїЎїЎ°Хд°®ЙъГьЈ¬ѕЬѕш¶ѕЖ·Ў±УИЖдКЗЗаЙЩДкёьУ¦Пт¶ѕЖ·ЛµЎ°І»Ў±Ј¬ИзОьКіЎ°±щ¶ѕ(јЧ»щ±Ѕ±ы°·ЈєC10H15N)Ў±іЙс«єу¶ѕё±ЧчУГєЬЗїЈ¬»бСПЦШЖЖ»µИЛµДЙъАнєНГвТЯ»ъДЬЎЈПВБРУР№ШЛµ·ЁІ»ХэИ·µДКЗ
A. јЧ»щ±Ѕ±ы°·КЗУР»ъОп B. јЧ»щ±Ѕ±ы°·µДПа¶Ф·ЦЧУЦКБїОЄ149
C. ГїёцјЧ»щ±Ѕ±ы°··ЦЧУє¬УР26ёцФЧУ D. јЧ»щ±Ѕ±ы°·ЦРCЎўHЎўNµДЦКБї±ИОЄ10:15:1
 »ЖёФМмМмБ·їЪЛгМвїЁПµБРґр°ё
»ЖёФМмМмБ·їЪЛгМвїЁПµБРґр°ё
| Дкј¶ | ёЯЦРїОіМ | Дкј¶ | іхЦРїОіМ |
| ёЯТ» | ёЯТ»Гв·СїОіМНЖјцЈЎ | іхТ» | іхТ»Гв·СїОіМНЖјцЈЎ |
| ёЯ¶ю | ёЯ¶юГв·СїОіМНЖјцЈЎ | іх¶ю | іх¶юГв·СїОіМНЖјцЈЎ |
| ёЯИэ | ёЯИэГв·СїОіМНЖјцЈЎ | іхИэ | іхИэГв·СїОіМНЖјцЈЎ |
їЖДїЈєіхЦР»ЇС§ АґФґЈє МвРНЈє
ЎѕМвДїЎїїЖС§µДєЛРДКЗСРѕїЈ¬їЖС§їОіМНЁ№эїЖС§МЅѕїµД·ЅКЅЈ¬ИГОТГЗЗЧАъїЖС§»о¶ЇЈ¬МеПЦїЖС§µД·ўПЦ№эіМєН·Ѕ·ЁЎЈ
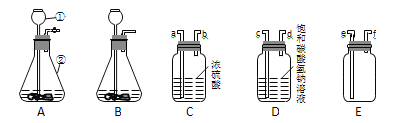
(ўс)ТСЦЄЈє¶юСх»ЇМјЖшМејИІ»ИЬУЪ±ҐєНNaHCO3ИЬТєТІІ»УлNaHCO3·ґУ¦Ј» NaHCO3 + HCl == NaCl + H2O + CO2ЎьЎЈДіС§ЙъУыУГЕЁСОЛбУлґуАнКЇ·ґУ¦ЦЖИЎёЙФпґїѕ»µД¶юСх»ЇМјЖшМеЈ¬КµСйКТМṩБЛПВБРЧ°ЦГєНТ©Ж·ЎЈЗл»ШґрПа№ШОКМвЈє
 ЎЎЎЎЎЎ
ЎЎЎЎЎЎ
(1)ТЗЖчўЩµДГыіЖКЗ__________________ЎЈ
(2)КµСйКТЦЖИЎ¶юСх»ЇМјЖшМеµД·ґУ¦ФАнКЗ___________________(УГ»ЇС§·ЅіМКЅ±нКѕ)ЎЈ
(3)Ч°ЦГAЎўB¶јїЙЧч·ўЙъЧ°ЦГЈ¬ЖдЦРїЙїШЦЖ·ґУ¦ЅшРРµДКЗ________(МоЧ°ЦГРтєЕ)ЎЈ
(4)БЅёцѕ»»ЇЧ°ЦГПаБ¬ЅУК±Ј¬ЖдТЗЖчЅУїЪЛіРтУ¦ОЄ______ЅУ______(МоЅУїЪРтєЕ)ЎЈ
(5)ИфУГЧ°ЦГEКХјЇ¶юСх»ЇМјЖшМеЈ¬ФтЖшМеУ¦ґУ______(МоЅУїЪРтєЕ)ЅшИлЎЈ
(ўт)ПВГжКЗіхИэДі°аС§ЙъМЅѕїМъЙъРвКµСйІ»¶ПНкЙЖУЕ»ЇµД№эіМЎЈ
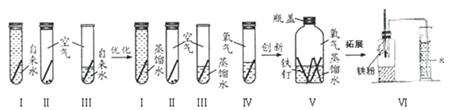
(1)УЕ»ЇКµСйК±Ѕ«ўсЦРЧФАґЛ®»»ОЄХфБуЛ®ДїµДКЗ_____________________________ЎЈ
(2)УЕ»ЇКµСйК±ФцјУўфКЗОЄЦ¤ГчМъЙъРвУлїХЖшЦРµД_____________(Мо»ЇС§КЅ)УР№ШЎЈ
(3)КµСйўхФЪИнЛЬБПЖїЦРНкіЙµДЈ¬Т»ЦЬєуїЙ№ЫІмµЅМъ¶¤ЙъРвЈ¬_____________Ј¬Н¬К±Мъ¶¤їїЅьЛ®±ЯёЅЅьІї·ЦРвКґµГАчє¦ЎЈУЙґЛїЙЦЄЈєМъЙъРвКµјККЗМъУл_______________(Мо»ЇС§КЅ)№ІН¬ЧчУГµДЅб№ыЎЈ
(4)КµСйўц»№їЙУГАґНкіЙ______________________(МоКµСйГыіЖ)ЎЈ
Ійїґґр°ёєНЅвОц>>
їЖДїЈєіхЦР»ЇС§ АґФґЈє МвРНЈє
ЎѕМвДїЎїДіРЛИ¤РЎЧйµДН¬С§¶ФТ»°ьѕГЦГµДЙъКЇ»Т(CaO)ёЙФпјБІъЙъБЛєГЖжЈ¬УЪКЗЛыГЗ¶ФХв°ьёЙФпјБµДіЙ·ЦХ№їЄБЛМЅѕїЎЈ
ЎѕМбіцОКМвЎїХв°ьёЙФпјБКЗ·с±дЦКЈ¬іЙ·ЦКЗКІГґ?
ЎѕІВПлјЩЙиЎїІВПлТ»ЈєИ«ІїКЗCaOЈ» ІВПл¶юЈєКЗCaOєНCa(OH)2µД»мєПОпЈ»
ІВПлИэЈєИ«ІїКЗCa(OH)2Ј» ІВПлЛДЈєКЗCa(OH)2єНCaCO3µД»мєПОпЎЈ
ЎѕКµСйМЅѕїЎї(1)ИЎІї·ЦёГёЙФпјБУЪКФ№ЬЦРЈ¬јУЛ®ОЮ·ЕИИПЦПуЈ¬ЛµГчёЙФпјБЦРІ»є¬ ЎЈ
(2)јМРшПтКФ№ЬЦРµОјУЧгБїПЎСОЛбЈ¬УРЖшЕЭіцПЦЈ¬ЛµГчХв°ьёЙФпјБЦРє¬УР ЎЈ
(3)ОЄБЛЅшТ»ІЅИ·¶ЁХв°ьёЙФпјБЦРУРОЮЖдЛыіЙ·ЦЈ¬РЎЧйН¬С§ЙијЖБЛТФПВ¶юЦЦ·Ѕ°ёЎЈ
БнИЎІї·ЦёЙФпјБУЪЙХ±ЦРЈ¬јУЛ®ІўЅБ°иЈ¬ѕІЦГєуИЎЙПІгЗеТєУЪ3Ц§КФ№ЬЦРЎЈЗлДгІОУлКµСйЈ¬ІўМоРґ±нЦРµДїХ°ЧЈє

Рґіц·Ѕ°ё¶ю·ўЙъµД»ЇС§·ґУ¦·ЅіМКЅ ЎЈ
ЎѕКµСйЅбВЫЎїНЁ№эТФЙПКµСйМЅѕїЈ¬µГіцІВПл іЙБўЎЈ
ЎѕНШХ№ЗЁТЖЎїРЎЧйН¬С§·ґЛјЙъКЇ»ТёЙФпјБ±дЦКФТтЈ¬ИПК¶µЅКµСйКТ±ЈґжСх»ЇёЖУ¦ЧўТв ЎЈ
Ійїґґр°ёєНЅвОц>>
їЖДїЈєіхЦР»ЇС§ АґФґЈє МвРНЈє
ЎѕМвДїЎїі¬ПёМјЛбёЖїЙУГУЪЙъІъёЖЖ¬ЎўСАёаµИІъЖ·ЎЈАыУГМј»Ї·ЁЙъІъі¬ПёМјЛбёЖµДЦчТЄБчіМКѕТвИзПВЈє

(1)КЇ»ТКЇµДЦчТЄіЙ·ЦµД»ЇС§КЅОЄ__________ЎЈ
(2)Ий»ЇіШЦРЈ¬ЙъКЇ»ТУлЛ®·ўЙъ·ґУ¦Ј¬Жд»ЇС§·ЅіМКЅОЄ________________ЎЈ
(3)№эЙёµДДїµДКЗА№ЅШ_______(МоЎ°ґуУЪЎ±»тЎ°РЎУЪЎ±)ЙёїЧЦ±ѕ¶µДїЕБЈЎЈ
(4)Мј»ЇіШЦР·ґУ¦µД»ЇС§·ЅіМКЅОЄ__________________________________ЎЈ
Ійїґґр°ёєНЅвОц>>
їЖДїЈєіхЦР»ЇС§ АґФґЈє МвРНЈє
ЎѕМвДїЎїИзНјКЗAОпЦКєНBОпЦКµДИЬЅв¶ИЗъПЯЈ¬»ШґрПВ БРОКМвЈє
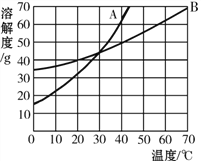
(1)ґУИЬЅв¶ИЗъПЯНјїЙ»сµГµДЦчТЄРЕПўКЗ(Рґ2Мх)Јє
ўЩ_________________________________________Ј¬
ўЪ_________________________________________ЎЈ
(2)ФЪОТГЗКмП¤µДОпЦКЦРЈ¬·ыєПНјЦРaµДИЬЅв¶ИЗъПЯµДОпЦККЗ____________ЎЈ
(3)40ЎжК±Ј¬Ѕ«30g BОпЦК·ЕИЛ50gЛ®ЦРЈ¬ід·ЦЅБ°иЈ¬ґЛК±ИЬЦКЦКБї·ЦКэКЗ________(ѕ«И·µЅ0.1%)Ј»ПЦЅ«ёГИЬТєЅµОВЦБ20ЎжЈ¬Оціц№ММе_________gЎЈ
Ійїґґр°ёєНЅвОц>>
їЖДїЈєіхЦР»ЇС§ АґФґЈє МвРНЈє
ЎѕМвДїЎїН¬С§ГЗФЪСйЦ¤ЗвСх»ЇёЖµД»ЇС§РФЦКК±Ј¬·Ц±рИЎЙЩБїЗвСх»ЇёЖИЬТєУЪЛДЦ§КФ№ЬЦРЈ¬ЧцБЛТФПВКµСйЈєЗлёщѕЭКµСй»ШґрПВБРОКМвЎЈ

(1)AЦР№ЫІмµЅµДПЦПуКЗ_______________ЎЈ
(2)РґіцDЦР·ўЙъ·ґУ¦µД»ЇС§·ЅіМКЅКЗ____________________________
(3)CЦРОЮГчПФПЦПуЈ¬ОЄЦ¤ГчЗвСх»ЇёЖУлСОЛбДЬ·ўЙъ·ґУ¦Ј¬ЛыГЗПтBЦРµОјУПЎСОЛбЈ¬µ±№ЫІмµЅ______К±Ј¬ЛµГчБЅХЯ·ўЙъБЛ·ґУ¦ЎЈ
(4)КµСйЅбКшєуЈ¬Н¬С§ГЗЅ«ЙПКцЛДЦ§КФ№ЬЦРµДОпЦКµ№ИЛН¬Т»ЙХ±ДЪЈ¬ЙХ±µЧІїУР°ЧЙ«№ММеЈ¬ЙПІгОЄОЮЙ«ИЬТєЈ¬ґЛК±ИЬТєЦРµДИЬЦКТ»¶ЁУР_______(іэ·УМЄНв)Ј¬їЙДЬУРµДИЬЦККЗЈє____ЎЈ
(5)ёщѕЭЗвСх»ЇёЖµД»ЇС§РФЦКЈ¬РґіцЛьµДТ»ЦЦУГНѕ______________ЎЈ
Ійїґґр°ёєНЅвОц>>
їЖДїЈєіхЦР»ЇС§ АґФґЈє МвРНЈє
ЎѕМвДїЎїёщѕЭЛщС§ЦЄК¶»ШґрПВБРОКМв
ЈЁ1Ј©ПВ±нКЗKNO3ФЪІ»Н¬ОВ¶ИК±µДИЬЅв¶ИЈ¬Зл»ШґрПВБРОКМвЎЈ

ўЩ·ЦОц±нЦРКэѕЭЈ¬№йДЙіцKNO3µДИЬЅв¶ИЛжОВ¶И±д»ЇµД№жВЙ___________ЎЈ
ўЪ20ЎжК±Ј¬ФЪЧ°УР5g KNO3ѕ§МеµДКФ№ЬЦРЈ¬јУИл10gЛ®Іўід·ЦХсµґЈ¬ґЛК±ЛщµГИЬТєОЄ___(СЎМоЎ°±ҐєНЎ±»тЎ°І»±ҐєНЎ±)ИЬТєЎЈФЩЅ«ёГКФ№Ь·ЕИлКўУРИИЛ®µДЙХ±ЦРЈ¬К№КФ№ЬДЪИЬТєОВ¶ИЙэЦБ60ЎжЈ¬ґЛК±КФ№ЬДЪµДИЬТєЦРИЬЦКУлИЬјБµДЦКБї±ИОЄ_______ЎЈ
ўЫЕдЦЖТ»¶ЁИЬЦКЦКБї·ЦКэµДKNO3ИЬТєК±Ј¬ИЬЅв№эіМЛщРиТЄµДТЗЖчУР______ЎЈ
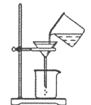
ЈЁ2Ј©ґЦСОµДіхІЅМбґїКµСйЎЈ
ўЩ іЖИЎ5.0gґЦСОЈ¬УГТ©іЧЦрЅҐјУИл10mLЛ®ЦРЈ¬Ц±µЅґЦСОІ»ФЩИЬЅвОЄЦ№Ј¬»№РиТЄЅшРРµДКµСйІЩЧчІЅЦиµДЛіРтОЄЈєіЖБїКЈУаґЦСОЎў_________ЈЁМо±аєЕЈ©ЎЈ
A.№эВЛ B.јЖЛгІъВК C. Хф·ў D. іЖБїѕ«СО
ўЪ№эВЛІЩЧчИзУТНјЛщКѕЈ¬ЦёіцЖдЦРµДґнОуЦ®ґ¦ЈЁРґіцБЅґ¦јґїЙЈ©Јє
__________________Ј»__________________
Ійїґґр°ёєНЅвОц>>
їЖДїЈєіхЦР»ЇС§ АґФґЈє МвРНЈє
ЎѕМвДїЎї1875ДкЈ¬·Ё№ъ»ЇС§јТІјНЯІ©µВАК·ўПЦБЛТ»ЦЦРВФЄЛШпШЈЁЖдФЄЛШ·ыєЕОЄGaЈ©Ј¬ЛьДЬУл·РЛ®ѕзБТ·ґУ¦ЙъіЙЗвЖшєНЗвСх»ЇпШЈ»пШµДФЧУЅб№№КѕТвНјИзНјЛщКѕЎЈЗл»ШґрПВБРОКМвЈє
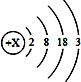
ЈЁ1Ј©пШФЄЛШФЧУµДЦКЧУКэОЄ_______________Ј»
ЈЁ2Ј©ёГФЄЛШФЪФЄЛШЦЬЖЪ±нЦРУлВБФЄЛШФЪН¬Т»ёц_____________ЈЁМоЎ°єбРРЎ±»тЎ°ЧЭРРЎ±Ј©Ј¬Жд»ЇС§РФЦКУлВБФЄЛШПаЛЖЈ¬ФТтКЗ_____________________________________Ј»
ЈЁ3Ј©ЗвСх»ЇпШУлЗвСх»ЇДЖ¶јѕЯУРјоµДРФЦКЈ¬ФтЗвСх»ЇпШУлПЎБтЛб·ґУ¦µД»ЇС§·ЅіМКЅОЄ________ЎЈ
Ійїґґр°ёєНЅвОц>>
їЖДїЈєіхЦР»ЇС§ АґФґЈє МвРНЈє
ЎѕМвДїЎїЗлёщѕЭПВБРКµСйЧ°ЦГ»ШґрОКМв:

(1)РґіцТЗЖчўЩўЪµДГыіЖЈєўЩ__________________Ј»ўЪ_____________________ЎЈ
(2)УГёЯГМЛбјШЦЖИЎСхЖшµД»ЇС§·ЅіМКЅОЄ________________________________Ј»µ±УГFЧ°ЦГКХјЇВъO2ІўИЎіцјЇЖшЖїєу,НЈЦ№ёГКµСйµДХэИ·ІЩЧч·Ѕ·ЁКЗ________________ЎЈ
(3)їЙУГEЧ°ЦГКХјЇµДЖшМеКЗ________________(РиґрБЅЦЦ)ЎЈ
(4)Бт»ЇЗв(H2S)КЗУР¶ѕЖшМе,КµСйКТіЈУГїйЧґБт»ЇСЗМъ(FeS)єНПЎБтЛбФЪіЈОВПВ·ґУ¦ЦЖИЎБт»ЇЗв(H2S)ЖшМе,У¦СЎУГµД·ўЙъЧ°ЦГЧоєГКЗ_________________ЎЈ
(5)јм–ЛCЧ°ЦГЖшГЬРФµД·Ѕ·ЁКЗ:ПИУГµЇ»ЙјРјРЧЎµјЖш№ЬЙПµДЅєЖ¤№Ь,ФЩПті¤ѕ±В©¶·ЦР јУИлЛ®_______________________,ѕІЦГЈ¬Иф№ЫІмµЅ________________,ЛµГчЖшГЬРФБјєГЎЈ
(6)GЧ°ЦГїЙУГУЪЖшМеµДКХјЇЎўјмСйЎўіэФУєНМе»эµДІвБїµИ,УГёГЧ°ЦГІ»ДЬНкіЙµДКµСйКЗ______________(МоРтєЕ)ЎЈ
A.ЖшМеґУa¶ЛНЁИл,КХјЇЗвЖшЎЈ
B.ЖїДЪЧ°УРіОЗеКЇ»ТЛ®,ЖшМеґУa¶ЛНЁИл,јмСйСхЖшЦРЦРКЗ·с»мУР¶юСх»ЇМјЎЈ
C.ФЪb¶ЛБ¬ЅУБїНІ,ЖїДЪЧ°ВъЛ®,СхЖшґУa¶ЛНЁИл,ІвБїЖшМеµДМе»эЎЈ
D.ЖїДЪЧ°УРЗвСх»ЇДЖИЬТє,ЖшМеґУa¶ЛНЁИл,ОьКХТ»Сх»ЇМјЦР»мУРµД¶юСх»ЇМјЎЈ
Ійїґґр°ёєНЅвОц>>
°Щ¶ИЦВРЕ - Б·П°ІбБР±н - КФМвБР±н
єю±±КЎ»ҐБЄНшОҐ·ЁєНІ»БјРЕПўѕЩ±ЁЖЅМЁ | НшЙПУРє¦РЕПўѕЩ±ЁЧЁЗш | µзРЕХ©ЖѕЩ±ЁЧЁЗш | ЙжАъК·РйОЮЦчТеУРє¦РЕПўѕЩ±ЁЧЁЗш | ЙжЖуЗЦИЁѕЩ±ЁЧЁЗш
ОҐ·ЁєНІ»БјРЕПўѕЩ±Ёµз»°Јє027-86699610 ѕЩ±ЁУКПдЈє58377363@163.com