
 ��2008?��ɽ��һģ��ij��ѧ��ѧѧϰС�����Ķ��ˡ���ȥ֯�������յķ��������ֲ����ܹ����ڳ�ȥˮ���ա�Ѫ�ա����⡢��īˮ�����գ��Բ��������Ũ�����Ȥ����ʦ����ͬѧ�Dz���Ļ�ѧʽΪH2C2O4�����ȷֽ⣮ͬѧ�ǶԲ������ȷֽ����̽����
��2008?��ɽ��һģ��ij��ѧ��ѧѧϰС�����Ķ��ˡ���ȥ֯�������յķ��������ֲ����ܹ����ڳ�ȥˮ���ա�Ѫ�ա����⡢��īˮ�����գ��Բ��������Ũ�����Ȥ����ʦ����ͬѧ�Dz���Ļ�ѧʽΪH2C2O4�����ȷֽ⣮ͬѧ�ǶԲ������ȷֽ����̽����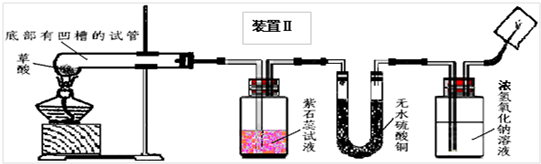
| ||
| ||
| ||
| ||



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
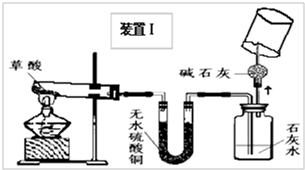
 ��2008?��ɽ��һģ�������������������ⶨ��ʵ��װ����ͼ������˵����ȷ���ǣ�������
��2008?��ɽ��һģ�������������������ⶨ��ʵ��װ����ͼ������˵����ȷ���ǣ��������鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
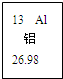 ��2008?��ɽ��һģ����ͼ��Ԫ�����ڱ��е�һ��Ԫ�أ������йظ�Ԫ�ص���Ϣ��ȷ���ǣ�������
��2008?��ɽ��һģ����ͼ��Ԫ�����ڱ��е�һ��Ԫ�أ������йظ�Ԫ�ص���Ϣ��ȷ���ǣ��������鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
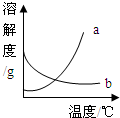 ��2008?��ɽ��һģ����ͼ��a��b�������ʵ��ܽ�����ߣ�����ʱ����ʢ��a��b������Һ���Թֱܷ�����ձ��ڴ������µ�ˮ�У������ձ��ڵ�ˮ�м�������粒����Ũ������Թ�����ʾ������ȷ���� ��������
��2008?��ɽ��һģ����ͼ��a��b�������ʵ��ܽ�����ߣ�����ʱ����ʢ��a��b������Һ���Թֱܷ�����ձ��ڴ������µ�ˮ�У������ձ��ڵ�ˮ�м�������粒����Ũ������Թ�����ʾ������ȷ���� ���������鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com