
| �� | �� | �� | �� | �� | �� | �� |
 |  |  |  |  |  | 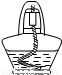 |
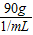 =90mL������90mL�������С����ͲΪ100mL����Ͳ���ʴ�Ϊ��100mL 90��
=90mL������90mL�������С����ͲΪ100mL����Ͳ���ʴ�Ϊ��100mL 90��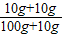 ×100%=18.2%����A����
×100%=18.2%����A���� ×100%=20%����B�ԣ�
×100%=20%����B�ԣ�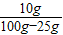 ×100%=13.3%����C����
×100%=13.3%����C����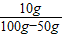 ×100%=20%����D�ԣ�
×100%=20%����D�ԣ�

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�����������ǵ䡱���������С����ɲ�������Ĵ�Ⱦ��һֱ���������࣬��������ѡ��ʹ���Լ���������̽�����о��ͳ������Ż��⡣�������������У�84����Һ���������ᣨCH3COOOH����Һ��˫��ˮ��Һ�����ᣨCH3COOH����Һ�ȣ�������ij��ͬѧ��չ�о���ѧϰ��������⣺
������1��Ϊ�˱����о���С������������������Է�������Ϊ__________��
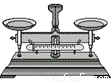
������2��С����С������ͬ�������Ĺ�������ʹ��ᣬ������ԭ�ӵĸ������Ƕ��٣�
����_________��
������3��С��������2%��˫��ˮ��Һ���Ѿ���4%��˫��ˮ��Һ100 g������ˮ������Ϊ____��
������4��С����100 gһ���������������Ĵ�����Һ������̼���Ʒ�Ӧ����Ӧ�Ļ�ѧ����ʽΪ��2CH3COOH+Na2CO3=2CH3COONa+H2O+CO2���������ɵ�����ͨ��������NaOHŨ��Һ�����NaOHŨ��Һ����4.4 g������������ԭ������Һ����������������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�����������ǵ䡱���������С����ɲ�������Ĵ�Ⱦ��һֱ���������࣬��������ѡ��ʹ���Լ���������̽�����о��ͳ������Ż��⡣�������������У�84����Һ���������ᣨCH3COOOH����Һ��˫��ˮ��Һ�����ᣨCH3COOH����Һ![]() �ȣ�������ij��ͬѧ��չ�о���ѧϰ��������⣺
�ȣ�������ij��ͬѧ��չ�о���ѧϰ��������⣺
������1��Ϊ�˱����о���С������������������Է�������Ϊ__________��
������2��С����С������ͬ�������Ĺ�������ʹ��ᣬ������ԭ�ӵĸ������Ƕ��٣�
����_________��
������3��С��������2%��˫��ˮ��Һ���Ѿ���4%��˫��ˮ��Һ100 g������ˮ������Ϊ____��
������4��С����100 gһ���������������Ĵ�����Һ������̼���Ʒ�Ӧ����Ӧ�Ļ�ѧ����ʽΪ��2CH3COOH+Na2CO3=2CH3COONa+H2O+CO2���������ɵ�����ͨ��������NaOHŨ��Һ�����NaOHŨ��Һ����4.4 g������������ԭ������Һ����������������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ��ʴ���

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2010�긣��ʡ�������谲������ѧҵ������黯ѧ�Ծ��������棩 ���ͣ������

�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com