
(2008������)����������װ��ͼ�ش����⣺
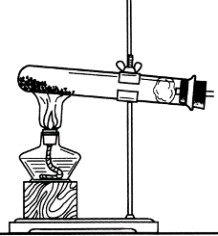
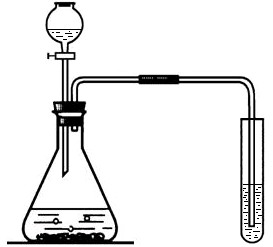
(1)д���б�����������ƣ�a____________��b____________��
(2)ʵ�����ü�װ���������Ļ�ѧ����ʽ��______________________________����____________���ռ���������Ӧ��������ȴ�����Թ��м���������ˮ�����衢���ˣ��õ���ɫ��ĩ���ú�ɫ��ĩ���������Ӵ��д������ݲ�������Ӧ�Ļ�ѧ����ʽ��____________________________________����ɫ��ĩ�ڷ�Ӧ�е�������____________��
(3)ij��ѧ��ȤС������װ����ȡ�����������̼��ʵ������У��ɹ۲쵽�Թ������____________ɫ��������Ӧ�Ļ�ѧ����ʽ��____________________________________���������������ͨ�������̼����һ��ʱ����ֳ����ܽ��ɳ�����Һ��Ϊ��ȷ�������ܽ�ɳ�����Һ��ԭ��С���ͬѧ���������̽����
������� ����Ϊʲô���ܽ��ɳ�����Һ��
�������� ̼���������ᣬ̼����ƣ�Ca(HCO3)2������ˮ��
��������� ����Һ�����ԣ��ڷ�Ӧ������̼����ơ�
ʵ�������
ʵ����� | ʵ������ | ʵ����� |
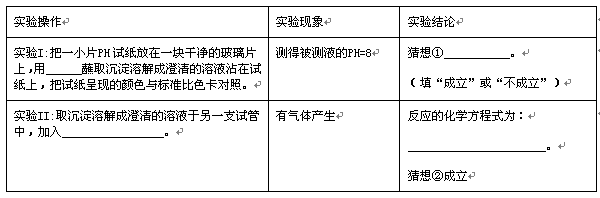
ʵ���һСƬpH��ֽ����һ��ɾ��IJ���Ƭ�ϣ���_________պȡ�����ܽ�ɳ������Һմ����ֽ�ϣ�����ֽ���ֵ���ɫ�����ɫ�����ա� | ��ñ���Һ��pH��8 | �����______________�� (���������������) |
ʵ���ȡ�����ܽ�ɳ������Һ����һ֧�Թ��У�����______________________ ___________________�� | ��������� | ��Ӧ�Ļ�ѧ����ʽΪ�� ______________________________________�� ����ڳ����� |
ͨ��̽����֪�����ɵij������������̼��ˮ��Ӧ�����˿�����ˮ��̼����ơ������뷴˼��̽������õ�����ʾ�������___________________________��

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�020
(2008������)�ֱ����и�������ͬʱ�ӵ�ˮ�У��ܴ���������ǡ������� ( )
A.NaCl��AgNO3��Na2SO4���������������� B.H2SO4��NaCl��Na2CO3
C.Na2SO4��KNO3��NaOH��������������������D.BaCl2��NaCl��KOH���� (˫ѡ)
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�058
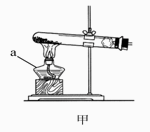
(2008������)����������װ��ͼ�ش����⣺
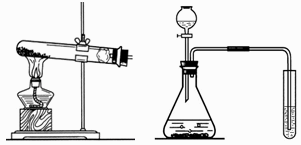
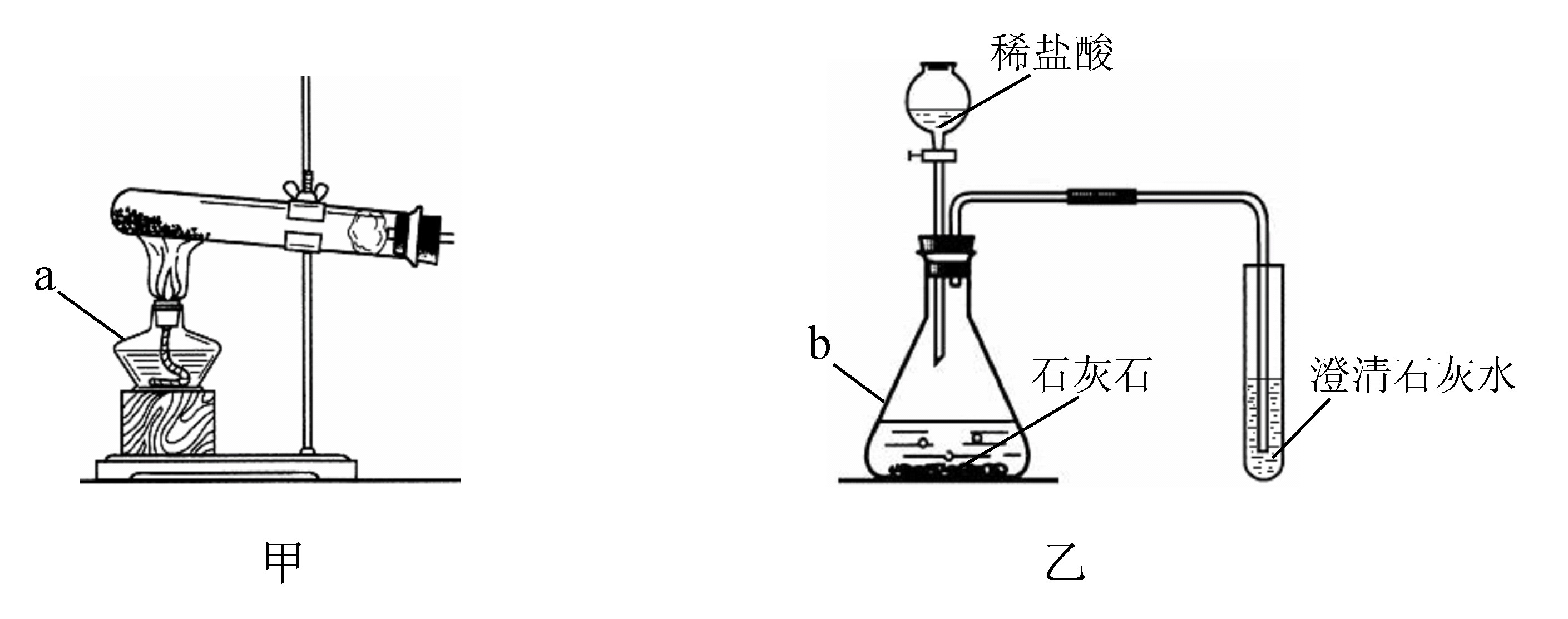
(1)д���б�����������ƣ�a____________��b____________��
(2)ʵ�����ü�װ���������Ļ�ѧ����ʽ��______________________________����____________���ռ���������Ӧ��������ȴ�����Թ��м���������ˮ�����衢���ˣ��õ���ɫ��ĩ���ú�ɫ��ĩ���������Ӵ��д������ݲ�������Ӧ�Ļ�ѧ����ʽ��____________________________________����ɫ��ĩ�ڷ�Ӧ�е�������____________��
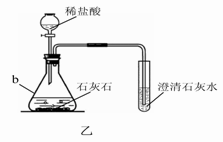
(3)ij��ѧ��ȤС������װ����ȡ�����������̼��ʵ������У��ɹ۲쵽�Թ������____________ɫ��������Ӧ�Ļ�ѧ����ʽ��____________________________________���������������ͨ�������̼����һ��ʱ����ֳ����ܽ��ɳ�����Һ��Ϊ��ȷ�������ܽ�ɳ�����Һ��ԭ��С���ͬѧ���������̽����
������⡡������Ϊʲô���ܽ��ɳ�����Һ��
�������ϡ���̼���������ᣬ̼����ƣ�Ca(HCO3)2������ˮ��
��������� ����Һ�����ԣ��ڷ�Ӧ������̼����ơ�
ʵ�������
ͨ��̽����֪�����ɵij������������̼��ˮ��Ӧ�����˿�����ˮ��̼����ơ������뷴˼��̽������õ�����ʾ�������___________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
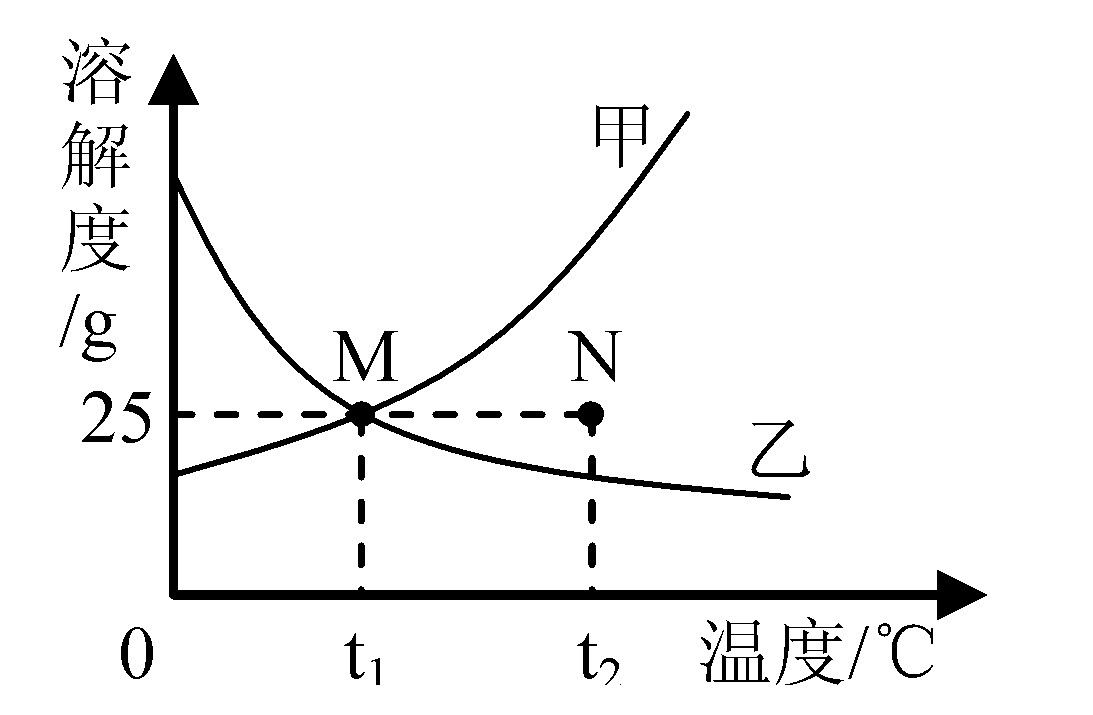
(2008������)ͼΪ�ס������ֹ���������ˮ�е��ܽ�����ߡ�����˵���������( )
A.�����ʵ��ܽ�����¶ȵ����߶�����
B.��t1��ʱ���ס��������ʵ��ܽ�����
C.��t2��ʱ��N���ʾ�����ʵIJ�������Һ
D.��t1��ʱ��100g�����ʵı�����Һ�����ʵ�������25g
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
(2008������)�ֱ����и�������ͬʱ�ӵ�ˮ�У��ܴ���������� ( )
A.NaCl��AgNO3��Na2SO4 B.H2SO4��NaCl��Na2CO3
C.Na2SO4��KNO3��NaOH D.BaCl2��NaCl��KOH (˫ѡ)
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com