
����̼���С�������ᴩ���Ϻ������Ľ����С�
��1������̼���С��Ľ�������˶�����̼������ŷţ��ܼ��� �ij̶ȣ���Ȼ�������Ķ�����̼����Ҫ;���� ������д��һ���ճ������з��ϡ���̼���á������������ ��
��2��������ѧ��PaulSabatier���á����ת��������ʹCO2��H2�ڴ�������������CH4��H2O����д���÷�Ӧ�Ļ�ѧ����ʽ ��
��3�������п�ѧ��������á�̼��������������ҵ�����ж�����̼���ŷ�������̼����������ָͨ��һ���ķ���������ҵ�����в�����CO2����������д�������á�������������NaOH��Һ��������CO2����������ͼ��ʾ����������������δ�������
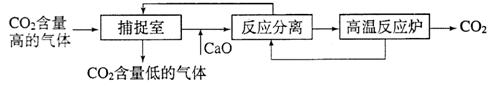 |
�ٲ����з�����Ӧ�Ļ�ѧ����ʽΪ�� ��
�ڰ�CaO���뷴Ӧ����������H2O��Ӧ����д���÷�Ӧ�Ļ�ѧ����ʽ�� �����ô˷�Ӧ�������ƿ�����ʳƷ ����
�ۡ���Ӧ���롱�У��õ��������ʵĻ��������� ���ù�����̼��ơ�
�����������У�����ѭ�����õ������� ��
��4��ȡ10g̼��ƹ�����¼��ȣ�һ��ʱ���ֹͣ���ȣ����ʣ������и�Ԫ�ص���������Ϊ50%���������ж���ȷ����
A.������2g������̼
B��ʣ���������Ϊ5g
C��������5.6g������
D��ʣ��̼��Ƶ�����Ϊ8g
 �����Ļ�������ҵϵ�д�
�����Ļ�������ҵϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| ||
| ||

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
| ||
| ||
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ��ʴ���
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com