
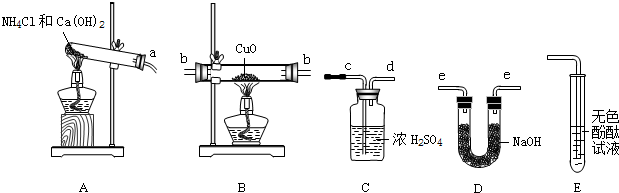
��ѧС����ݰ�����ԭ����ͭ�ķ�Ӧ�����ʵ��ⶨCuԪ�ص����ԭ����������֪���� 2NH4Cl+Ca(OH)2=CaCl2+2NH3��+2H2O �� ������NH3���Ǽ�������
������ͼ�ش����⡣

��1������������İ���ͨ��B�У��۲쵽�������ں�ɫ�����Ϊ����ɫ���ܿ���Һ�Σ�ͬʱ���ɿ����к����������壬д��B�з�����Ӧ�Ļ�ѧ����ʽΪ ��
��2���ⶨCuԪ�����ԭ��������ʵ�����Ϊ���ȳ���CuO����������ȫ��Ӧ��ⶨ����ˮ���������ɴ˼����CuԪ�ص����ԭ��������
��.С��ͬѧ������ͼ����ʵ�飬����װ�����Ӻ������ǣ�����ţ�װ�ÿ��ظ�ʹ�ã� ��
�� ACBDC �� ADBCD �� ADBDC �� ABDC
��.�ڱ�ʵ���У�ʹ�ⶨ���ƫ���ԭ�������_______________ (�����)��
�� CuOδ��ȫ��Ӧ �� CuO������
�� CuO�л��в���Ӧ������ �� NH4C1��Ca(OH)2����ﲻ����
��.�ڱ�ʵ���У���ͨ���ⶨ___________________________�������ﵽʵ��Ŀ�ġ�
����������1�����ݰ�����ԭ����ͭ��Ӧ�������жϷ�Ӧ�������д����Ӧ�Ļ�ѧ����ʽ�����п����к�����������Ϊ������
��2���ٸ���ʵ�����룬�����ṩ��װ����ѡ���������װ����װ��ʵ��ʵ��Ŀ��ʵ��װ�ã�
�ڷ������ṩ���ض�ʵ������Ӱ�죬�ж����лᵼ�²ⶨ���ƫ������أ�
�۸��ݶ�ʵ������⣬Ϊ�ﵽ�ⶨĿ�ģ��жϻ����Բ�ȡ�IJ���ֵ��

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

| ||
| ||
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012�챱���г������п���ģ��ѧ�Ծ����������� ���ͣ�̽����
��ѧС����ݰ�����ԭ����ͭ�ķ�Ӧ�����ʵ��ⶨCuԪ�ص����ԭ����������֪���� 2NH4Cl+Ca(OH)2=CaCl2+2NH3��+2H2O �� ������NH3���Ǽ�������
������ͼ�ش����⡣
��1������������İ���ͨ��B�У��۲쵽�������ں�ɫ�����Ϊ����ɫ���ܿ���Һ�Σ�ͬʱ���ɿ����к����������壬д��B�з�����Ӧ�Ļ�ѧ����ʽΪ ��
��2���ⶨCuԪ�����ԭ��������ʵ�����Ϊ���ȳ���CuO����������ȫ��Ӧ��ⶨ����ˮ���������ɴ˼����CuԪ�ص����ԭ��������
��.С��ͬѧ������ͼ����ʵ�飬����װ�����Ӻ������ǣ�����ţ�װ�ÿ��ظ�ʹ�ã� ��
�� ACBDC �� ADBCD �� ADBDC �� ABDC
��.�ڱ�ʵ���У�ʹ�ⶨ���ƫ���ԭ�������_______________ (�����)��
�� CuOδ��ȫ��Ӧ �� CuO������
�� CuO�л��в���Ӧ������ �� NH4C1��Ca(OH)2����ﲻ����
��.�ڱ�ʵ���У���ͨ���ⶨ___________________________�������ﵽʵ��Ŀ�ġ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012�걱���г������п���ѧ��ģ�Ծ��������棩 ���ͣ������

�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com