��κ�������Ч����Լ��ʹ��ú��ʯ�͡���Ȼ����������������Ҫ�Ļ�ʯȼ�ϣ��ӽ��������������������Դ��ȫ�������ٵĹ�ͬ���⣬�Ƿ�չ��̼���õĵ���֮�����ش��������⣺
��1����Ȼ������ɽ���м�ͥ����Ҫ����ȼ�ϡ�Ϊ��ֹ����Ȼ��й©���Σ�գ����ڼ��а�װ���������ڼס�����ͼ�У���װλ����ȷ����________��д����Ȼ��ȼ�յĻ�ѧ����ʽ___________��
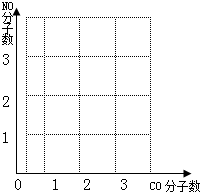
��2����ֱ����ú������ȼ�ϼ���Ⱦ�������˷���Դ��úȼ��ʱ���������γ��������Ҫ����֮һ��ú�������ǰ�ú��Ϊ�����Դ����Ҫһ��������һ����Ҫ��Ӧ�ǣ�C+H
2O

CO+H
2���÷�Ӧ�Ļ���������_____________��
��3���ҹ������з���һ�ֵ綯����ʹ�õ���Դ������﮵�أ��ӻ�ѧ�Ƕȿ�����ʹ�����͵�����������ŵ��ǣ��ٳ�һ�㼴�ɣ�_________________��

 CO+H2���÷�Ӧ�Ļ���������_____________��
CO+H2���÷�Ӧ�Ļ���������_____________��

 ��κ�������Ч����Լ��ʹ��ú��ʯ�͡���Ȼ��������ʯȼ�ϣ��ӽ��������������������Դ��ȫ�������ٵĹ�ͬ���⣬�Ƿ�չ��̼���õĵ���֮�����밴Ҫ��ش��������⣮
��κ�������Ч����Լ��ʹ��ú��ʯ�͡���Ȼ��������ʯȼ�ϣ��ӽ��������������������Դ��ȫ�������ٵĹ�ͬ���⣬�Ƿ�չ��̼���õĵ���֮�����밴Ҫ��ش��������⣮ ��κ�������Ч����Լ��ʹ��ú��ʯ�͡���Ȼ����������������Ҫ�Ļ�ʯȼ�ϣ��ӽ��������������������Դ��ȫ�������ٵĹ�ͬ���⣬�Ƿ�չ��̼���õĵ���֮�����ش��������⣺
��κ�������Ч����Լ��ʹ��ú��ʯ�͡���Ȼ����������������Ҫ�Ļ�ʯȼ�ϣ��ӽ��������������������Դ��ȫ�������ٵĹ�ͬ���⣬�Ƿ�չ��̼���õĵ���֮�����ش��������⣺