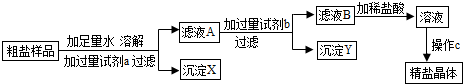
�⣺��1�������������������Һ��Ŀ����Ϊ�˳�ȥ�����е��Ȼ�þ����Ϊ�������ƺ��Ȼ�þ��Ӧ������������þ�������Ȼ��ƣ�
����Ȼ�þ��
��2���ڹ��˲����У����õ�����Ȧ������̨���ձ����������⣬����Ҫ�õ���������©����
���©����
��3��50g������������Ϊ6%��NaCl��Һ�У���������Ϊ��50g��6%=3g��ˮ�������ǣ�50g-3g=47g��ˮ���ܶ���1g/ml������ˮ������ǣ�47g��1g/ml47ml��
���3��47��
��4������ҺB�μ�ϡ����Ĺ����У�ϡ�����Ⱥ������������Ʒ�Ӧ������������ȫ��Ӧ��ϡ�����ٺ�̼���Ʒ�Ӧ�����۲쵽�ո������ݲ���ʱ��˵��ϡ����ǡ�ú�̼������ȫ��Ӧ����Ϊ�����ӷ�����ʹ���������Ҳ����Ӱ�쾫�ξ���ijɷ֣�
����ո������ݲ�����û�У�
��5������C������������ʱ��һЩҺ�塢���彦����������������ʳ�ξ���ת�Ƴ���ʱ��һ������������������ʱ�����ܹ�ʹ���þ��α�����ͬѧ����Ҫ�٣����ᴿ�����þ�����δ��ȫ�������ʹ���þ��α�����ͬѧ����Ҫ�࣮
���������AB��
��6����ⱥ��ʳ��ˮ�Ļ�ѧ����ʽΪ��2NaCl+2H
2O

2NaOH+H
2��+Cl
2����
���2NaCl+2H
2O

2NaOH+H
2��+Cl
2����
��������1���������ƺ��Ȼ�þ��Ӧ������������þ�������Ȼ��ƣ�
��2������ʱ���õ��������д���Ȧ������̨���ձ�����������©����
��3��������Һ�����������������Լ��������������ܼ�������
��4�������ӷ�����̼���Ʒ�Ӧ�������Ȼ��ơ�ˮ�Ͷ�����̼��
��5�����������������³��ֽϴ����
��6�����ݷ�Ӧ��������Ӧ�������������غ㶨�ɿ�����д��ѧ����ʽ��
��������ѧʵ�������ǻ�ѧʵ����ͻ�����������IJ��֣�Ҳ�ǽ��з��������ó����۵����ݣ��������ʵ����ʺ��֮��ķ�Ӧ��ϵ����������߹۲�ʵ�顢����ʵ������������ԣ��Ի�ѧʵ�鲻��Ҫ����۲죬��Ӧ�������ʵ�顢�۲�ʵ������ķ�����

 2NaOH+H2��+Cl2����
2NaOH+H2��+Cl2���� 2NaOH+H2��+Cl2����
2NaOH+H2��+Cl2����

 һŵ��ҵ�����ҵ���ּ�����������������ϵ�д�
һŵ��ҵ�����ҵ���ּ�����������������ϵ�д�





